Acute kidney injury (AKI) describes a sudden loss of kidney function, and it usually affects people who are already hospitalized.
AKI tends to occur as a result of another illness or medicine, although the condition can also affect healthy people.
Estimates show that approximately “two-thirds of critically ill patients” develop AKI, which raises their risk of death above 60 percent.
Moreover, the incidence of AKI is on the rise. According to the National Institutes of Health (NIH), the rate of AKI cases that need dialysis has risen by 10 percent each year over the last decade. The number of AKI-related deaths has also doubled, the NIH report.
So, the need for more effective AKI treatments is urgent, and new research brings us closer to developing such therapies.
In AKI, kidney tissue cannot heal, which leads to kidney dysfunction. However, scientists at the University of Alabama at Birmingham (UAB) are working to find new ways to promote healing.
Dr. Anupam Agarwal, director of the Division of Nephrology in the UAB Department of Medicine, together with James George, Ph.D., a professor at the UAB Department of Surgery, led the new study. It has been published in the journal JCI Insight.
The research team found that immune cells called macrophages revert to a developmental state during AKI. These cells could be used to drive the healing of kidney tissue.

How macrophages reprogram post-AKI
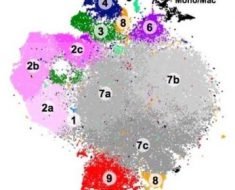
To examine the types of cells that can be found in damaged kidneys, the researchers used a process called parabiosis — in which they join together the cardiovascular systems of two organisms, in this case, two mice.
The team did this to determine whether the macrophages that they found in the kidneys post-AKI resulted from other cells having invaded the kidneys in response to damage or if they derived from “kidney-resident macrophages” that renewed themselves.

The scientists joined the rodents’ circulatory systems for a period of 4 weeks, during which they triggered AKI in one of the mice by inducing “bilateral ischemia/reperfusion.”
The immune cells of the rodents had different markers, which enabled the researchers to track the cells that invaded the kidneys following AKI.
Dr. Agarwal and the team observed that the invading cells contributed very little to the kidney-resident macrophages found in the kidneys after AKI.
Therefore, “the renewing source of [kidney-resident macrophages] after AKI is predominantly in situ renewal, as opposed to infiltration of macrophage precursors from the blood,” conclude the authors, who also detail some of the mechanisms that may explain the findings.
Kidney-resident macrophages, note the researchers, “undergo transcriptional reprogramming toward a developmental state following injury.” This reprogramming leads to expressing a gene profile that is similar to those of kidney-resident macrophages in 7-day-old mice.
The immune cells also had greater levels of Wnt signaling. Researchers understand this pathway to be key for the development of the kidneys in mice and humans.

Implications for new AKI therapies
Concerning the findings, Jeremie M. Lever, one of the study’s first authors, comments, “macrophage biology has reached a pivotal point.”
“Many basic science research studies have suggested the importance [of] tissue-resident macrophages in healing after injury, but [the] development of therapies promoting them is still in early stages,” he continues.
“In order to successfully utilize these cells for contemporary translational interventions, [we need] to be specific about the origin — tissue-resident versus infiltrative — of the cells we plan to target,” explains Lever.
Co-first author Dr. Travis D. Hull, Ph.D., says, “This work demonstrates that tissue-resident macrophages possess the same plasticity that has been demonstrated in other immunological cell types.”
“Moreover, this ability to reprogram to an early ontological phenotype is a potential avenue for therapeutic intervention, if the cellular signals and mechanisms of this reprogramming can be fully elucidated.”
Dr. Travis D. Hull, Ph.D.
“This is an exciting development in the field of [AKI],” Hull says, adding that it “also may represent a therapeutic target in fields such as transplantation, where the importance of macrophage biology is less well-understood.”
Source: Read Full Article