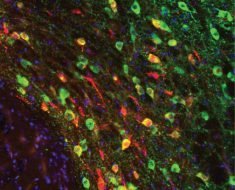
The protein BRAF is a key player in the development of many different types of cancer, including melanoma. Scientists have known that BRAF becomes activated by growth factors and subsequently stimulates downstream proteins that promote cancer cell growth, invasion and survival. However, less is understood about how BRAF is involved in communicating signals from pro-inflammatory cytokines that are released by immune cells in the surrounding tumor environment. Researchers in Moffitt Cancer Center’s Donald A. Adam Melanoma and Skin Cancer Center of Excellence have discovered a signaling pathway between cytokines and BRAF that promotes tumor growth.
Proteins in cells communicate to one another through direct and indirect mechanisms, and their activity is highly regulated by a variety of different types of protein modifications that can stimulate or inhibit their activity. One type of protein modification, ubiquitination, which typically serves as a tag for the cell to degrade that protein, has been shown to stimulate cell signaling, DNA damage responses, or cellular protein uptake. When these highly regulated modification pathways are altered, cells are more prone to become tumorigenic.
In a new study published this week by Nature Communications, Moffitt researchers outlined experiments in melanoma cell lines and mice to determine how communication signals from the immune system converge on BRAF and the consequences of these signals. They found that pro-inflammatory cytokines released from immune cells stimulate the protein ITCH to modify BRAF by ubiquitination. This ubiquitination modification prevents BRAF from interacting with inhibitory proteins, thereby maintaining the activity of BRAF and allowing it to stimulate the downstream proteins that promote cancer growth and metastasis.
These observations suggest that ITCH modification of BRAF may play an important role in cancer development. The researchers confirmed through experiments where the ITCH gene was deleted from cells. ITCH-deficient cells had reduced levels of BRAF ubiquitination and lower levels of active downstream proteins. The team also discovered that preventing BRAF ubiquitination reduced tumor growth in mice, suggesting that blocking ITCH activity or BRAF ubiquitination may be an effective treatment approach.
Source: Read Full Article