A vaccine originally developed to prevent bacteria-caused diarrhea has now also been found to help infant nonhuman primates grow faster, according to a new study published in Nature Communications.
“The 160 million people worldwide who get sick every year from Campylobacter bacteria is far too many,” said the study’s lead researcher, Mark Slifka, Ph.D., a professor at Oregon Health & Science University’s Oregon National Primate Center. “We need a new tool to prevent bacterial diarrhea in babies and to enable more children to grow into healthy adults, and this vaccine approach looks very promising.”
The study evaluated a vaccine that uses a hydrogen peroxide-based technology called HydroVax, which Slifka developed at OHSU. The university licensed the technology to Najít Technologies, Inc., where Slifka serves as the company’s president and chief scientific officer. The technology is also helping develop vaccines against other diseases like yellow fever, West Nile and the flu.
Campylobacter-associated diarrhea is often mild, but can be fatal in young children, older adults or people with suppressed immune systems. Although Campylobacter illness is more common in developing countries, the Centers for Disease Control and Prevention estimates that the bacteria sickens 1.5 million people in the United States every year. It is spread by consuming raw or undercooked poultry, drinking untreated water or being in contact with animal feces.
Infant growth stunting occurs when children grow at a slower than normal pace, and may be caused by a combination of poor nutrition and repeated intestinal infections. It can lead to poor health outcomes, as well as reduced earning potential in adulthood. The World Health Organization estimates that five or more incidents of diarrhea before age 2 is the primary cause for growth stunting in a quarter of children.
Similar to what has been documented in the wild, Campylobacter naturally circulates among outdoor-housed rhesus macaques at the Oregon National Primate Research Center. Slifka and colleagues vaccinated both pregnant monkeys and their babies for this study, and compared the health and growth rates of vaccinated and unvaccinated baby monkeys.
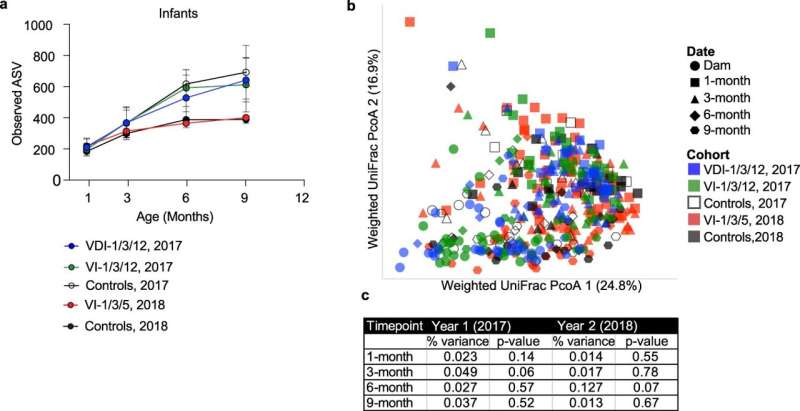
The researchers found that vaccinated baby monkeys were taller than the unvaccinated when measured from head to toe. To assess this, they specifically used LAZ, or Length-for-Age Z score, which is used to measure human children’s height relative to their age. They found the greatest difference was at 9 months of age, when vaccinated monkeys had a significant LAZ improvement of 1.28. In contrast, a recent study that reviewed the outcomes of 29 clinical trials involving human infants showed that growth stunting interventions either failed to provide any significant improvement in length or, at most, provided a LAZ increase of only 0.2. Based on these comparisons, Slifka and colleagues concluded their vaccine-based approach provides a substantial improvement over what other research on infant growth stunting prevention has been able to achieve.
After receiving the first two vaccine doses, about 55% of all vaccinated baby monkeys were protected from severe diarrhea, and up to 79% were protected when both infant and mother were vaccinated. That’s a decrease from the 83% vaccine efficacy that was found in an earlier Campylobacter vaccination study of adult monkeys. Slifka said the differences may be due to changes in which Campylobacter strain is naturally circulating among the center’s nonhuman primates, and because about 80% of the infants were already infected with Campylobacter prior to their first vaccination.
Importantly, the study also found that none of the vaccinated infants contracted a lethal Campylobacter infection, and that the vaccine reduced diarrhea-associated deaths for all causes—including bacteria other than Campylobacter—by 76%. This finding suggests that vaccination against this one common intestinal bacteria may also help reduce overall diarrhea-associated deaths.
“If the vaccine could be tailored to closely match the current circulating strains, administered at an earlier age, or if infants were not exposed to Campylobacter at such a young age, then there is a possibility that this vaccine approach could have even more profound effects on infant health and growth,” Slifka said.
Another interesting and unexpected finding was that infants born to vaccinated mothers had a significantly higher body mass index, or BMI, at one month of age compared with infants born to unvaccinated mothers. Because all of the adult monkeys had the same diet and all of the infants were exclusively breastfed, the researchers concluded that this result indicated vaccinating mothers during pregnancy provided an advantage to their infants at an early age. This result appears to be similar to the benefit of providing human mothers the DTaP vaccine, which provides more than 90% protection against infant whooping cough, also known as pertussis, during the first two months of life.
To build on the study’s findings, Slifka would like to explore further improving the vaccine’s effectiveness by creating a multivalent shot that fights against more than one strain of Campylobacter. Additionally, he would like to test the use of improved nutritional supplements alongside vaccination to determine if the combination further improves infant growth trajectories.
More information:
Sara M. Hendrickson et al, Campylobacter vaccination reduces diarrheal disease and infant growth stunting among rhesus macaques, Nature Communications (2023). DOI: 10.1038/s41467-023-39433-1
Journal information:
Nature Communications
Source: Read Full Article