A Japanese research team led by the National Cerebral and Cardiovascular Center Research Institute has identified for the first time progenitor cells that specifically transform into the contractile cells of the left ventricle during heart development. Identifying one of the diverse progenitor cells involved in the formation of the heart will guide future studies and enable a better understanding of congenital heart diseases.
The heart is a four-chamber organ containing various types of cells, each of which undergoes a different journey during embryologic development to reach its final form. Researchers in Japan have now advanced our understanding of this multifaceted process, which can shed light on the mechanisms underlying congenital heart disease and why heart-related problems can be so diverse.
To appreciate their research achievement, we first need to know a little about the development of the heart. Cardiomyocytes are the major contractile cells of the heart. They form the muscular walls of the atrium and ventricle—two chambers at each side of the heart. Atrial and ventricular cardiomyocytes have different characteristics, and each develops their unique identity at a very early embryonic stage.
This process takes place through the precise orchestration of special genes and communicator chemicals. We already know that there are precursor cells—called progenitor cells—that give rise to both atrial and left ventricular cardiomyocytes, but whether there is a specific, unique group of cells that transform into the left ventricular cardiomyocytes remained unclear—until now.
In an article published in the Proceedings of the National Academy of Sciences (PNAS), a Japanese research team led by the National Cerebral and Cardiovascular Center Research Institute addressed this question.
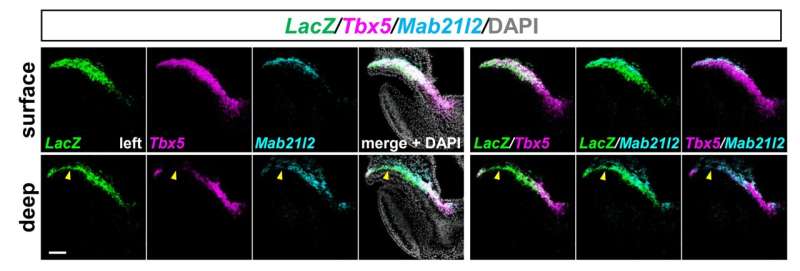
“We performed experiments on early mouse embryos and identified, for the first time, a group of progenitor cells that develop specifically into left ventricular cardiomyocytes. These cells are active in the early formation of the heart and show high activity of an enhancer of a gene called ‘Hey2’,” explains lead author Yusuke Watanabe.
In the early phase, these newly identified Hey2 enhancer-active cells are located right next to the already known common progenitor cells; they then overlap with each other, suggesting that the progenitor cells for left ventricular cardiomyocytes are initially heterogenous.
Senior author Osamu Nakagawa underlines the importance of this diversity, explaining, “These heterogenous, complex progenitor cells must be finely orchestrated to successfully form each part of a multi-chambered heart. Understanding how exactly this is achieved will provide clues to the mechanisms underlying the diversity of congenital heart diseases.”
The newly identified progenitor cells improve our understanding of the diversification of cell types during early cardiac development. These insights open up future work into how Hey2 enhancer-active cells are regulated, which could potentially improve the outlook of patients with congenital heart diseases affecting the left ventricle.
More information:
Yusuke Watanabe et al, Hey2 enhancer activity defines unipotent progenitors for left ventricular cardiomyocytes in juxta-cardiac field of early mouse embryo, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2307658120
Journal information:
Proceedings of the National Academy of Sciences
Source: Read Full Article