A research team of the Department of Medicine and Life Sciences (MELIS) at Pompeu Fabra University (UPF) involving the Hospital del Mar Research Institute has for the first time, in mice, identified and validated the neurobiological mechanism and therapy to correct memory deficit in individuals with fetal alcohol spectrum disorders (FASD). These results pave the way for studying whether the mechanism is the same in humans, which would enable improving the diagnosis and treatment of affected individuals.
Fetal alcohol spectrum disorder (FASD) includes a number of conditions suffered by infants who have been exposed to alcohol during pregnancy. The effects of FASD range from craniofacial morphological malformations or growth problems, in the most severe cases, to hyperactivity, emotional and motivational difficulties or defects in learning and memory, in the mildest cases.
“In children of normal appearance, FASD is underdiagnosed and is often mistaken for hyperactivity or ADD,” explains Rafael de la Torre, coordinator of the Integrated Pharmacology and Systems Neuroscience Research Group at the Hospital del Mar Research Institute. “Since there is no diagnosis, there is no treatment, and symptomatic therapy is given to alleviate hyperactivity or other disorders such as anxiety.”
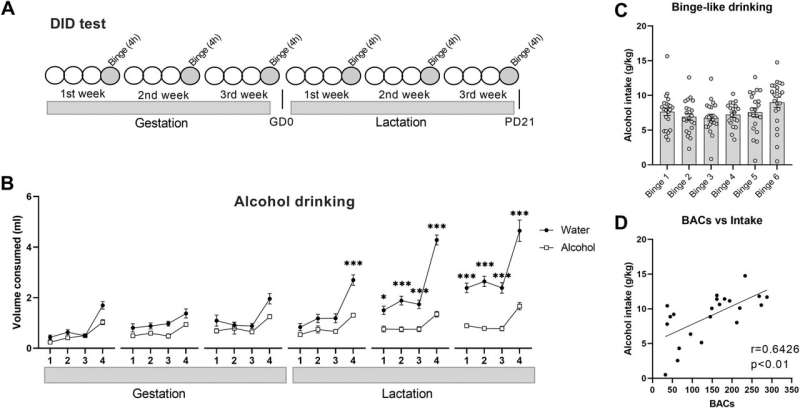
The results of the study published in the journal Molecular Psychiatry, have allowed the researchers to observe that “exposure to alcohol need not be chronic for FASD to occur. Sporadic consumption ending in intoxication—getting drunk- is enough to observe alterations in memory, in mice,” explains Olga Valverde, study coordinator and director of the Research Group in Behavioral Neurobiology at the MELIS-UPF.
The study shows that mice born to mothers that have consumed alcohol sporadically during pregnancy and lactation have a memory deficit that persists into adulthood. One of the reasons for this deficit is that alcohol affects the function of the endocannabinoid system, reducing the expression of the PPAR-? receptor.
“The endocannabinoid system is greatly involved in learning and memory processes,” de la Torre explains. “That is why it is especially relevant that this decrease occurs during infancy, when the mice, male and female, are of learning age.” However, the reduction in PPAR-? does not occur throughout the brain. It is limited to the hippocampal astrocytes -cells that support neurons controlling functions such as their metabolism or the inflammation to which they are subjected- of the hippocampus.
After confirming the neurobiological mechanism by three different routes, the study also proposes an effective treatment with the drug pioglitazone, commonly used to control sugar and which stimulates PPAR receptors. According to the first author of the study, Alba Garcia-Baos, “manages to alleviate the cognitive memory deficits of individuals with FASD in infancy.”
Optimism regarding studies in humans
The results of this study pave the way for studying the effects of other cognitive impairments caused by alcohol exposure during pregnancy. “In this work we have only studied alterations in memory, but there may be emotional, motivational or behavioral alterations related to FASD,” points out Valverde, who is also a full professor of Psychobiology at UPF.
Although this study has been conducted in mice, the researcher is optimistic about confirming that this mechanism is replicated in humans “because we are two species of mammals that share many similarities.” In addition, if confirmed, “it would be relatively simple to carry out a study to validate whether the therapy we propose works in humans, since there are drugs that have similar effects to the ones we have used that are approved for use in children.”
More information:
Alba Garcia-Baos et al, The role of PPAR-γ in memory deficits induced by prenatal and lactation alcohol exposure in mice, Molecular Psychiatry (2023). DOI: 10.1038/s41380-023-02191-z
Journal information:
Molecular Psychiatry
Source: Read Full Article