Many ocular diseases involve changes in the structure and function of different regions of the back of the eye, also known as the “eye fundus.” For example, fluorescent pigments and tiny yellowish deposits called drusen accumulate under the retina in age-related macular degeneration, and the degeneration of neurons called ganglion cells is a defining characteristic of glaucoma.
Interestingly, changes in the eye fundus are not restricted to vision-related diseases only. Certain neurological diseases like Parkinson’s and Alzheimer’s can cause changes in retinal nerves and blood flow.
In general, eye care professionals rely on color imaging and computed tomography techniques to diagnose ocular diseases. However, over the past few decades, scientists have found that disease-related changes in the eye fundus also modify its profiles for spectral reflectance and emittance. In other words, the way in which light interacts with specific retinal structures at different frequencies can provide important diagnostic information to complement standard imaging methods.
As a result, spectral analysis tools and techniques for light reflected or emitted by the eye fundus have steadily gained traction. Unfortunately, even though many different methods have been proposed, they still suffer from important limitations.
One common issue is that most spectroscopy-based methods can only make measurements over a large region of the eye fundus, which hinders their ability to detect fine spectral changes in small retinal structures. On the other hand, techniques that can make localized spectral measurements require the fixation of the patient, which can be very tedious and uncomfortable.
To tackle these issues, a research team from Zilia Inc., Canada, led by Professor Dominic Sauvageau from University of Alberta, has developed a much more flexible system for targeted spectroscopy in the eye fundus. In their study, published in Journal of Biomedical Optics (JBO), they present the rationale behind their design and demonstrate its potential through a series of comprehensive experiments.
The proposed system has a handful of key features that make it stand out as a more versatile and flexible alternative to existing ones. The entire device has a series of optical elements that essentially allow three different light paths to and from the eye fundus to coexist without hampering each other. More specifically, illumination LEDs, a color camera, and a spectrometer can be used simultaneously to provide continuous color imaging and spectral measurements.
Moreover, the spectrometer section of the device focuses an LED onto a small region of the eye fundus, and the position of this region can be adjusted using simple mechanical actuators to rotate the beam splitter feeding the camera and the spectrometer.
“The user can select a target and move it to any location within the eye fundus region being imaged without any realignment or change of the fixation target while continuously receiving spectral information of the targeted sampled area,” says Sauvageau.
This feature makes it easy to take spectral measurements from very specific anatomical structures, such as the optic nerve, the retina, leakage of blood, fat deposits or any type of lesion. Notably, the system can also be used to conduct fluorescence measurements by adjusting the illumination source, which extends its applicability to detect an even wider variety of biomarkers.
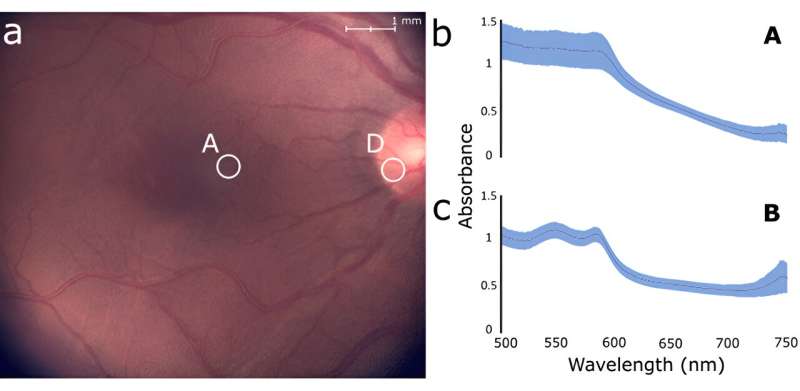
The research team confirmed the capabilities and performance of their system through both in vitro and in vivo experiments. The in vitro experiments involved targeting colored areas in a reference target with a grid-like pattern and taking spectral measurements of an eye model that simulated a macula, blood vessels, a foreign body, and the optic nerve. On the other hand, the in vivo measurements were performed on the retinas of eight healthy subjects, which exhibited differences in the spectral profiles of the optic nerve and the parafoveal region.
Taken together, the results of this study highlight the many advantages of the proposed design and could pave the way to better diagnostic protocols for eye diseases.
“Targeted ocular spectroscopy has the potential to assess the presence of different chromophores and fluorophores, such as hemoglobin, oxyhemoglobin, melanin, and lipofuscin, associated with disease progression,” says Sauvageau.
“This could open the door to changes in the way we diagnose and treat eye diseases, and targeted ocular spectroscopy could become an increasingly important tool in eye care in the coming years.”
More information:
Nicolas Lapointe et al, Targeted spectroscopy in the eye fundus, Journal of Biomedical Optics (2023). DOI: 10.1117/1.JBO.28.12.126004
Journal information:
Journal of Biomedical Optics
Source: Read Full Article