Whether arising from being felled on the soccer pitch or a seemingly harmless collision with a coffee table, a minor injury to the cartilage in your knee can have major consequences. In the worst case, the weak spot gives rise to severe arthritis and an artificial knee is the only hope. However, if the problem is caught early, further deterioration could be prevented by a patch repair. Doctoral candidate Meike Kleuskens developed a “training device” to test how such cartilage implants behave under considerable strain.
Your knees have to put up with a lot. Even gentle walking subjects the knee to a constant barrage of forces: compression, stretching, shearing. So the flexible cartilage in the knee joint has an ingenious structure, explains Meike Kleuskens. “Thanks to the unique curvature of the elastic collagen fibers in the cartilage, the forces are spread evenly throughout the tissue. That’s not something we can simulate with the current generation of implants.”
Even a relatively minor injury to the cartilage impairs this distribution of forces, causing tissue damage to occur elsewhere in the knee joint. Cartilage then starts to wear away and eventually even the surrounding bone can become damaged. “At some point the entire joint must be replaced by an artificial knee,” tells Kleuskens. “But that is major surgery, something you don’t want to perform more than once.” Yet, unfortunately, artificial knees last only fifteen to twenty years. And so it becomes advisable to postpone the operation for as long as possible, especially in view of people’s increasing longevity.
Growing tissue
This explains why ways to repair tissue damage immediately are eagerly being sought worldwide, to prevent it later having such devastating effects. Since no synthetic implants with the right properties exist as yet, hope is pinned on tissue engineering. This involves growing pieces of new tissue from the body’s own cells—in this case cartilage cells—supported by a synthetic yet biodegradable framework (or ‘matrix’). This cultivation can take place in the lab or within the body; once the implant is in place, tissue develops naturally within it as the matrix gradually decays.
The situation is further complicated by the fact that the knee has to be capable of withstanding such great forces. Experience shows that when the implant fails to integrate sufficiently with the surrounding tissue, the surgery outcome is often poor. Moreover, it takes months before the implant is, as it were, ‘fully grown’ and can actually withstand these forces, Kleuskens explains.
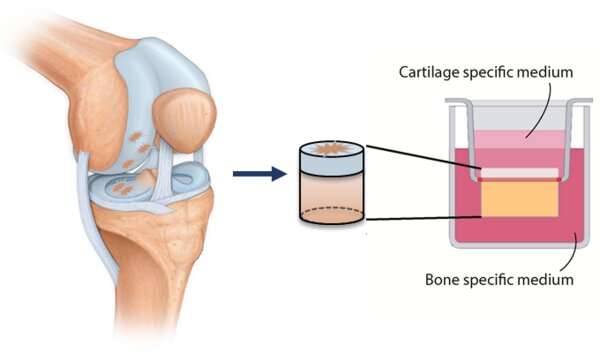
In her doctoral project in the Orthopedic Biomechanics group at the Department of Biomedical Engineering she therefore studied the circumstances most conducive to growing implants and integrating them in existing cartilage. Her research was based on cylindrical ‘plugs’ of cartilage on a disc of bone measuring a centimeter in diameter.
But unfortunately, cartilage cells don’t always do what you expect them to. Cells cultured in the usual way, in a dish, often make the wrong type of collagen, tells the doctoral candidate from Venlo. “This is why these implants are often not a success, especially in older patients.” Instead, the team at Orthopedic Biomechanics succeeded in producing three-dimensional pieces of tissue in cell culture flasks containing pieces of matrix material. These organoids, as they are called, measuring roughly 0.25 millimeters in diameter, she then implanted in the cartilage plugs.
The cartilage cells that Kleuskens used had been taken from knees removed from patients. In other words, cartilage from someone’s damaged joint, usually an older person. The question arose whether this is the right source material from which to culture vital cartilage tissue.
“And so I compared our culture results with results involving cartilage cells from UMC Utrecht, taken from younger, healthy people. I found no difference between that tissue and patient tissue, so that choice seems a good one. It is, moreover, material that is in plentiful supply and would otherwise go to waste.”
Massage
To make the situation as realistic as possible, she devised a way to expose the plugs containing fresh implants to strong forces. Together with the departmental spinoff LifeTec Group BV, she developed a training device of sorts, which gives the plugs a massage by means of a pair of pistons with smoothly polished heads (shown on the photo). “This device enables us to simulate a person walking.” For a whole month, because that’s how long it takes before cartilage cells in a new environment start to form a collagen matrix.
Source: Read Full Article