A team of medical researchers and doctors affiliated with multiple institutions in the U.S. has found, via clinical trial, that the aldosterone synthase inhibitor lorundrostat is likely safe to use for reducing blood pressure in patients with uncontrolled hypertension.
In their paper published in JAMA, the group describes the parameters of their clinical trial and the outcomes they observed. Bryan Williams, with University College London, has published an Editorial in the same journal issue outlining the history of research involving aldosterone and the work done by the team on this new effort.
Most people who have chronic high blood pressure (hypertension) can be treated with a handful of drugs that have proven to be effective. Unfortunately, there are some people who do not respond well to such drugs and who therefore need an alternative therapy. Such a therapy is critical for these patients, because without one, they will likely experience a heart attack or stroke. In this new effort, the research team used an aldosterone synthase inhibitor called lorundrostat to treat such patients.
Aldosterone is a hormone that signals the kidneys to absorb sodium and helps to regulate water and salt levels in the body. And that in turn plays a significant role in blood pressure regulation. For that reason, scientists have been looking for a drug that can be used to control the amount of aldosterone produced by the body as a way to control blood pressure. To that end, the team on this new effort used lorundrostat and tested it for both its efficacy and safety.
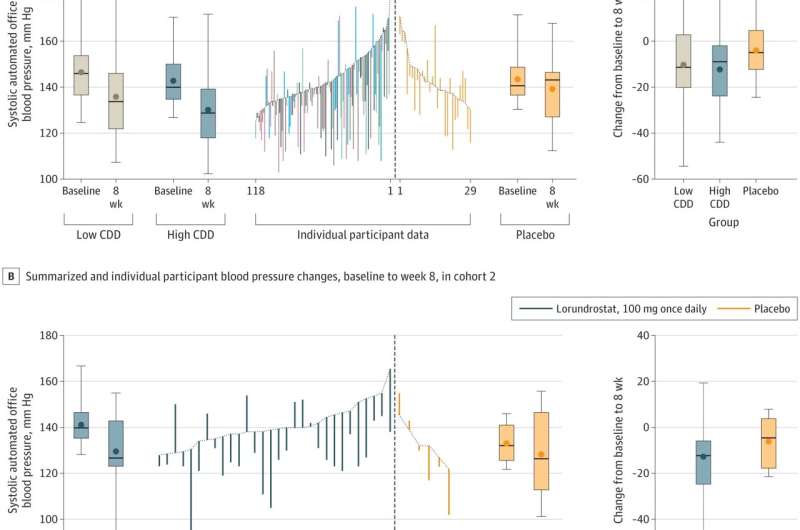
In their phase II clinical trial, the research team recruited 163 volunteers diagnosed with uncontrolled hypertension and who had suppressed renin production (an enzyme in the kidneys that promotes production of angiotensin). They were administered 50 or 100 mg of lorundrostat or a placebo every day for eight weeks. All the volunteers were tested regularly to determine if the drug was having an effect.
The research team found that those receiving the test drug saw an average systolic drop of 14.1 mm Hg by the end of the trial. They also noted that weight appeared to impact effectiveness—those who were more obese tended to see a bigger drop in their blood pressure. They also found no effect on cortisol synthesis and serum potassium levels increased by the amount expected. Thus, the drug has proven to be safe enough to proceed with a phase III trial.
More information:
Luke J. Laffin et al, Aldosterone Synthase Inhibition With Lorundrostat for Uncontrolled Hypertension, JAMA (2023). DOI: 10.1001/jama.2023.16029
Bryan Williams, A New Dawn for Aldosterone as a Therapeutic Target in Hypertension, JAMA (2023). DOI: 10.1001/jama.2023.17087
Journal information:
Journal of the American Medical Association
Source: Read Full Article