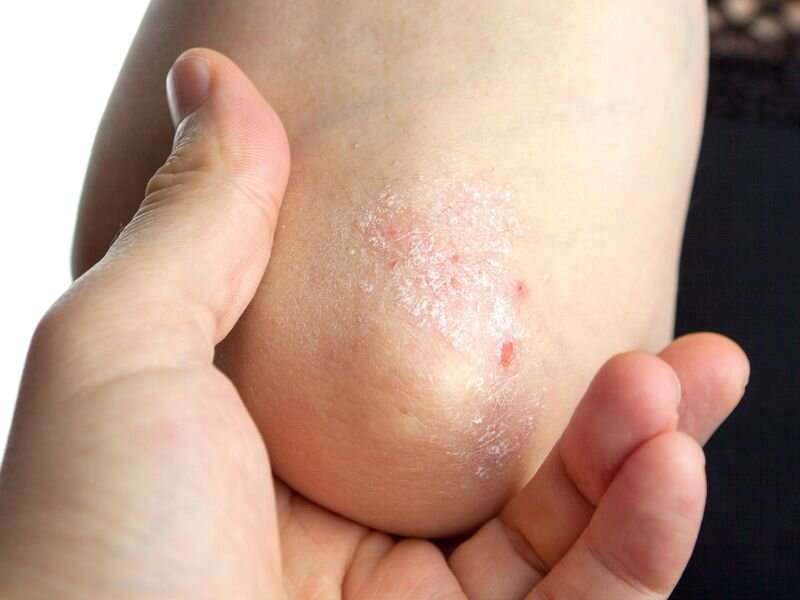
Guselkumab is consistently effective across subpopulations for the treatment of moderate-to-severe plaque psoriasis, according to a study published June 1 in the Journal of the European Academy of Dermatology & Venereology.
Knut Schäkel, M.D., from Heidelberg University Hospital in Germany, and colleagues evaluated the impact of early disease intervention on clinical responses following 28 weeks of guselkumab treatment in patients with moderate-to-severe plaque psoriasis. The analysis included 880 patients with a short disease duration (SDD; no more than two years; 40.6 percent) or a long disease duration (LDD; more than two years) receiving guselkumab 100 mg at weeks 0, 4, 12, 20, and 28.
The researchers found that more patients with SDD achieved absolute Psoriasis Area and Severity Index (PASI) scores of 0 at week 28 (51.8 versus 39.4 percent among LDD patients) and were super responders with complete skin clearance from weeks 20 to 28 (43.7 versus 28.1 percent). The median time to PASI of 0 was shorter among SDD patients than LDD patients (141 versus 200 days). Disease duration and prior biologic use were not independently associated with super response status. Across all patient groups, guselkumab was associated with a rapid decrease in markers of systemic inflammation at week 4.
“The proportion of [super responders] was higher in SDD than LDD patients, indicating early treatment intervention may improve clinical outcomes,” the authors write.
Several authors disclosed ties to pharmaceutical companies, including Janssen-Cilag, which manufactures guselkumab and funded the study.
More information:
K. Schäkel et al, Early disease intervention with guselkumab in psoriasis leads to a higher rate of stable complete skin clearance (‘clinical super response’): Week 28 results from the ongoing phase IIIb randomized, double‐blind, parallel‐group, GUIDE study, Journal of the European Academy of Dermatology and Venereology (2023). DOI: 10.1111/jdv.19236
Copyright © 2023 HealthDay. All rights reserved.
Source: Read Full Article