A new editorial paper titled “Subpopulations of AIB1 isoform-expressing breast cancer cells enable invasion and metastasis” has been published in Oncotarget.
In their new editorial, researchers Amber J. Kiliti, Ghada M. Sharif, Anton Wellstein, and Anna T. Riegel from Georgetown University Medical Center discuss potential mechanisms of breast cancer invasion and metastasis. Genetic and epigenetic events drive individual tumor cells to proliferate and expand into a heterogeneous mixture of cells that evade immune surveillance, acquire the ability to invade the vasculature and spread as metastatic seeds to distant sites. Organ metastasis contributes to more than 90% of all cancer-related deaths.
“The model of Darwinian evolution explains the stepwise selection of cancer cells capable of invasion and metastatic spread and an extensive body of work supports that cancer cell-autonomous features match the selected cancer cell ‘seed’ with the appropriate ‘soil’ of the target organ,” the researchers write.
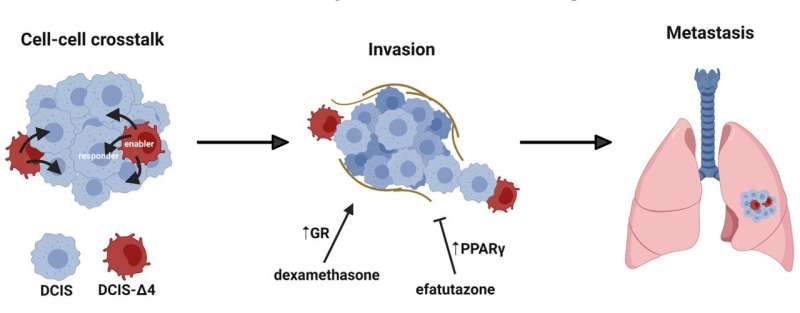
However, this concept was challenged in a recent paper in Cancer Research. Sharif et al. observed that a subclonal population of cells in a heterogeneous tumor can significantly alter the growth characteristics, invasiveness and metastasis of an entire tumor through cell-cell crosstalk. These functionally relevant cell subpopulations are difficult to detect through bulk analysis though their presence may influence disease outcome and efficacy of treatments.
The researchers explain, “In their paper, Sharif et al. detailed how expression of a splice isoform of the transcriptional coregulator and oncogene Amplified In Breast Cancer 1 (AIB1) in a small subpopulation of cells can lead to increased tumor growth and invasion of surrounding tissues by ductal carcinoma in situ (DCIS) cells.”
More information:
Amber J. Kiliti et al, Subpopulations of AIB1 isoform-expressing breast cancer cells enable invasion and metastasis, Oncotarget (2023). DOI: 10.18632/oncotarget.28452
Journal information:
Cancer Research
,
Oncotarget
Source: Read Full Article