In the 1835 Hans Christian Andersen fairy tale “The Princess and the Pea,” a princess is deemed authentic because of her sensitivity to a pea placed under 20 mattresses on her bed. New research from the McKelvey School of Engineering at Washington University in St. Louis has found that like the fabled princess, cancer cells can sense a layer of cells beneath the top collagen layer on which they normally travel, while normal cells cannot.
Amit Pathak, an associate professor of mechanical engineering and materials science, and Christopher Walter, a postdoctoral research associate in Pathak’s lab, found that cancer cells have what they term “depth mechanosensing,” or the ability to sense the properties of the distant environments underneath their immediate extracellular matrix. This could mean that the mechanical properties of extracellular matrices located away from the cells could regulate cell migration. Results of their research were published online in Cell Reports on April 5.
Pathak and his team have long studied the way cells migrate to determine how cancerous tumors invade tissue, how wounds heal and how organs and tissues develop. They have previously shown that cells move faster on stiffer environments due to higher forces and tractions than on soft environments.
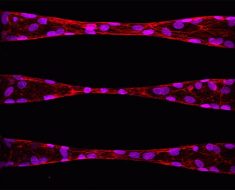
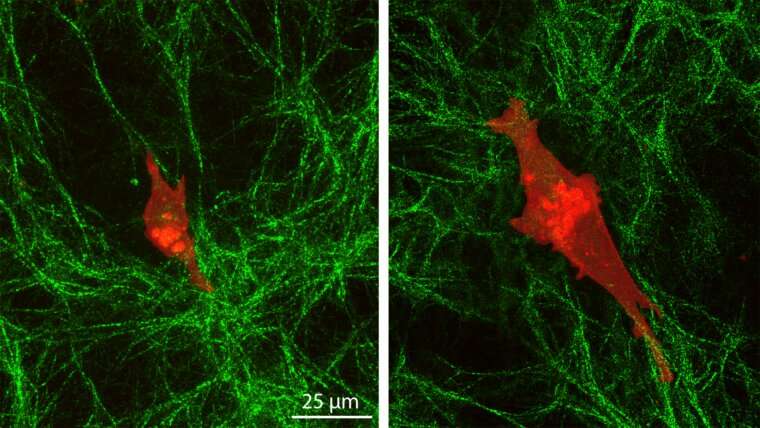
In the new work, Pathak and Walter created two hydrogels—one stiff and one soft—and added a layer of collagen-based extracellular matrix on top. Using a wide variety of techniques, including atomic force microscopy, high resolution imaging, and analyses of cell migration dynamics, they monitored the behavior of human mammary normal epithelial cells and triple-negative breast cancer cells on the surfaces.
Normal cells showed little change in shape or size and moved slowly on top of the collagen layer. The cancer cells on the stiff underlying gel elongated and extended more stable protrusions, which help them communicate across matrix layers, migrated faster and generated greater pulling of collagen fibers due to their depth mechanosensing.
“Cells sense deep into their environment, and their polarity installs a polarity in the extracellular matrix according to what the cell is telling it,” Pathak said. “These behaviors suggest that the shapes of the cancer cells are influenced by the stiffness of the underlying gel, while normal cells respond only to the immediate, soft collagen gel. This is something that was not known before.”
The cells’ polarity also helps to generate force for the cells to move in a particular direction, Walter said, but they also need the ability to pull on collagen fibers and modify their environments as it is friendlier for migration.
“We were able to get rid of their ability to sense the distant matrix by cutting the collagen itself or cross-linking it, eliminating the feedback that the matrix gives the cells,” Walter said. “If you lose either mechanism, the feedback stops, so they have to have both.”
Pathak said this feedback from the surface is important to the cell. Jairaj Mathur, a doctoral student in mechanical engineering & materials science, developed a computational model to integrate polarized forces of cells with collagen remodeling to explain the deep sensing observed in experiments.
“Our model shows that if you don’t have this feedback between the cells and the matrix, then this depth sensing may happen for a short time, but it won’t be sustained,” he said. “And this is why cancer cells are good at this, because they can recruit the matrix into migrating and depth sensing.”
More information:
Christopher Walter et al, Reciprocal intra- and extra-cellular polarity enables deep mechanosensing through layered matrices, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112362
Journal information:
Cell Reports
Source: Read Full Article