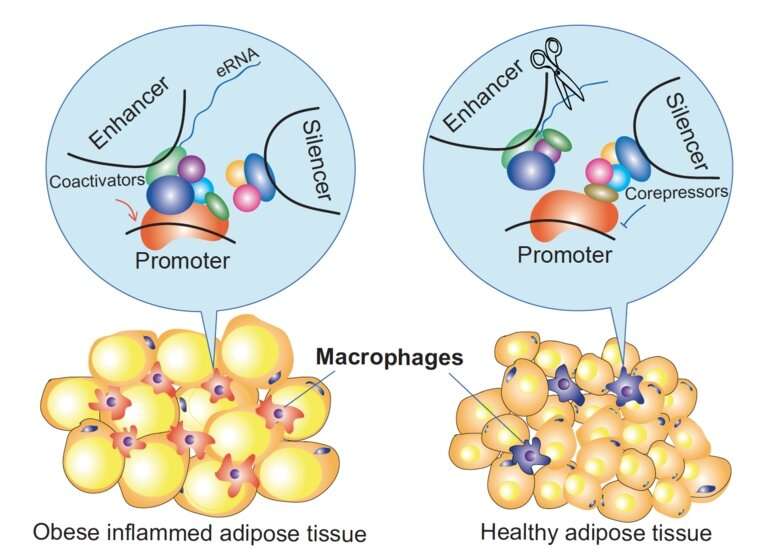
How do cis-regulatory genome elements regulate gene expression, what are the critical components involved, and can we therapeutically target them? By investigating how corepressors modulate enhancers and silencers during inflammatory macrophage activation, BioNut researchers have found some unexpected answers to these fundamental questions. The study is published today in Molecular Cell.
The laboratory of Eckardt Treuter, Professor of Molecular Cell Biology at the Department of Biosciences and Nutrition at KI, has a long-standing interest in understanding the mechanistic and physiological roles of transcriptional corepressors. Particularly their study in the context of macrophages has uncovered some epigenetic mechanisms that potentially underly the development of immuno-metabolic diseases, including type 2 diabetes and atherosclerosis. The researchers have previously found that one subunit of a fundamental corepressor complex, named GPS2, is downregulated in monocytes and macrophages of humans with obesity and type 2 diabetes. This probably affects the corepressor complex in many ways and results in alterations of inflammatory gene expression, but the underlying mechanistic details remained enigmatic.
In the current study, the researchers used an in vitro macrophage model to investigate chromatin structure and histone modifications, transcription factor and coregulator dynamics, and enhancer RNA transcription along with gene expression, in response to pro-inflammatory signaling.
Essential for chromatin remodeling
“Our study sheds light on the rather surprising dual role of corepressors at enhancers and silencers, cis-regulatory elements that control gene expression in seemingly opposite ways,” says Eckardt Treuter.
“We believe that corepressors, along with the transcription factors that bind to these elements, are essential for the signal-dependent chromatin remodeling within defined 3-D structures, so-called TADs.”
The study also provides new evidence that enhancer-transcribed eRNAs may have a functional role, an issue which is currently debated in the field. For example, corepressors and eRNAs seem to act antagonistically to concomitantly modulate 3-D chromatin organization and gene expression. The researchers could even reduce the macrophage expression of CCL2 (aka MCP-1, a major inflammatory chemokine) in mice, by depleting an eRNA of the critical Ccl2 enhancer using a modified short nucleic acid.
“We are particularly excited as our work demonstrates that macrophage eRNAs can be selectively targeted to reduce inflammation and thereby to improve metabolic health in obese mice,” says Assistant Professor Rongrong Fan, co-corresponding author.
Source: Read Full Article