In many neurodegenerative disorders, abnormal proteins progressively aggregate and propagate in the brain. But what comes first, aggregation or propagation? Researchers from Japan share some new insights about the mechanism involved in Parkinson’s disease.
In a study published recently in Cell Reports, researchers from Tokyo Medical and Dental University (TMDU) have shown that a mutated version of a protein called α-synuclein propagates to various cerebral regions through the lymphatic system and then aggregates.
Although the function of α-synuclein is not fully understood, it participates in neurotransmission. However, in some neurodegenerative diseases including Parkinson’s disease, α-synuclein changes shape and forms pathological clumps.
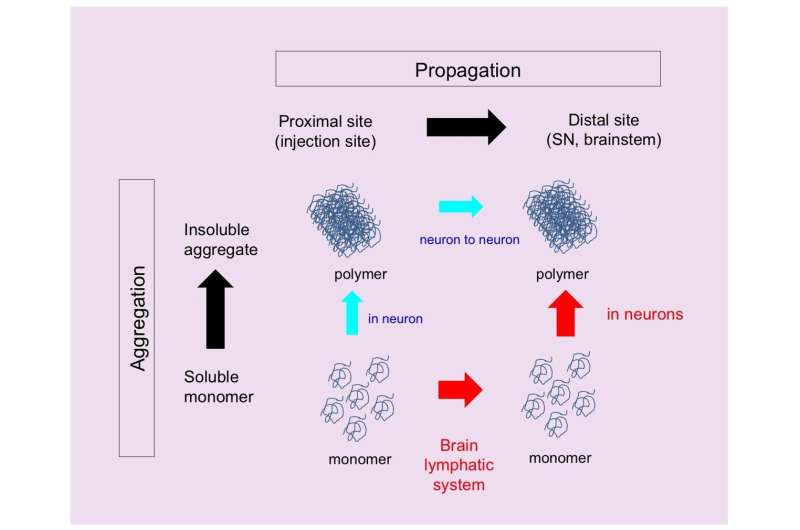
“Most experiments conducted so far only used fibrils, which are the clumps formed when monomeric α-synuclein aggregates. The fibrils are transmitted from neurons to neurons, but it remains unclear whether monomers act in the same way,” explains Kyota Fujita, an author of the study.
To further investigate how monomers and fibrils of α-synuclein move around in the brain, the researchers injected small amounts of viral particles into the orbital cortex of mice to produce fluorescent monomeric mutant α-synuclein. Because any cell type can contribute to α-synuclein propagation, they used viral particles to enable the synthesis of α-synuclein monomers in all cell types present in the injection area. This method ensured that all modes of propagation were accounted for.
Twelve months after the injection, although the fluorescent signal was lower in the injected region, signals were detected in other brain areas. Interestingly, fluorescent α-synuclein was detected in remote regions two weeks after injection, indicating an early spreading of mutant α-synuclein in the brain.
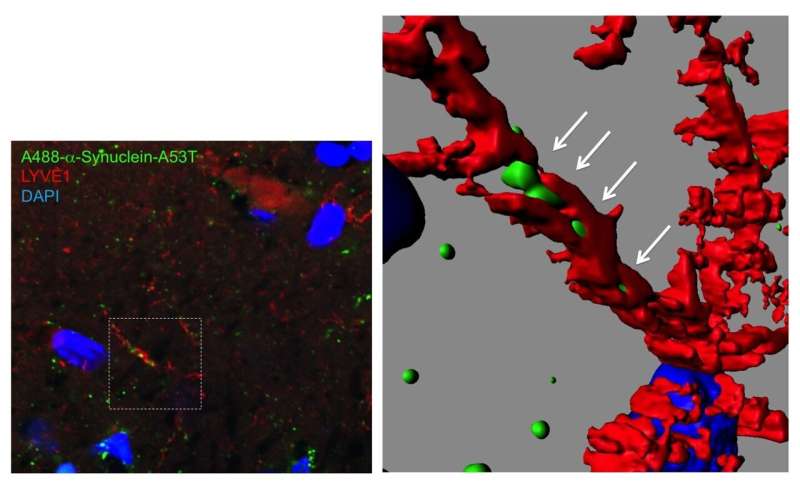
But how did α-synuclein propagate? The team followed the three-dimensional distribution of α-synuclein in the brain and found fluorescent α-synuclein in the glymphatic system, which is the lymphatic system of the brain. The glymphatic system is involved in draining and renewing fluid from the brain and eliminating toxins, but it could also distribute toxic substances throughout the brain.
The team also observed the presence of fluorescent α-synuclein in the matrix surrounding neurons and in the cytosol of neurons. This finding suggested that fluorescent α-synuclein was taken up by the extracellular matrix and, subsequently, by neurons.
The researchers also investigated the aggregation state of α-synuclein in the remote brain regions. “Fibrils of α-synuclein formed after the monomers had propagated,” says Professor Hitoshi Okazawa, the research group leader. “Specifically, we observed α-synuclein monomer in the glymphatic system and remote regions as early as two weeks after injection, while we found α-synuclein fibrils 12 months after injection.”
The amount of α-synuclein aggregated and the time at which they formed after injection varied among regions and was not proportional to the distance from the injection site. This observation is consistent with the known vulnerability of some regions to pathological α-synuclein.
This study shows how monomeric α-synuclein propagates through the glymphatic system in a different way from the fibrils. Thus, targeting these early events, α-synuclein monomer and brain lymphatic system, may limit the progression of Parkinson’s disease.
More information:
Kyota Fujita et al, Mutant α-synuclein propagates via the lymphatic system of the brain in the monomeric state, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112962
Journal information:
Cell Reports
Source: Read Full Article





