New research published today in Nature Genetics describes the largest-ever study into the genetics of random “round-the-clock” blood glucose levels. The study, involving almost half a million people of diverse backgrounds, describes new DNA variants influencing blood sugar levels measured “at random.” The team of researchers, led by Professor Inga Prokopenko at the University of Surrey on behalf of the Meta-Analysis of Glucose and Insulin-related Traits Consortium (MAGIC), analyzed data from 17 major studies, including the UK Biobank.
This research highlighted that individual responses to drugs from the popular class of GLP-1R agonists, used to treat type 2 diabetes and obesity, can depend on DNA variants in the target gene, GLP1R.
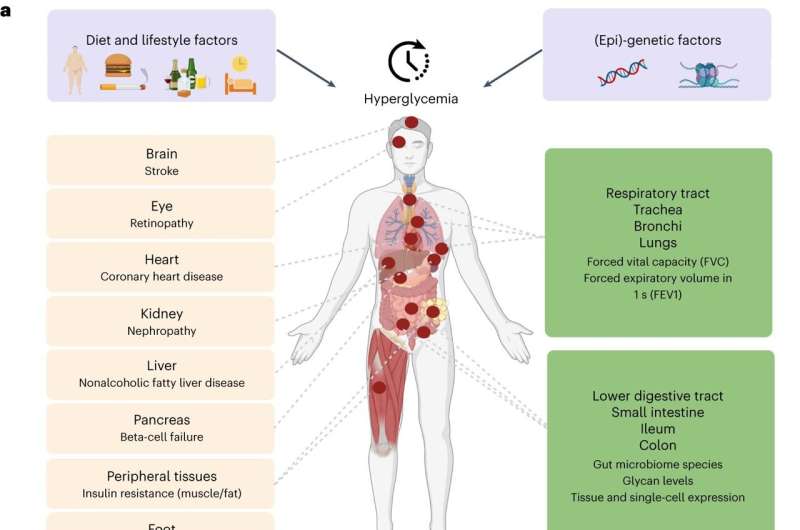
Scientists also revealed, for the first time, that type 2 diabetes can directly cause lung complications. In the largest-ever genetic study exploring how genes affect blood sugar levels and health outcomes, researchers concluded that lung disorders should now be considered a complication of type 2 diabetes.
The study combining genetic and expression data shed light on the importance of the gastrointestinal tract, where the small intestine, ileum, and colon play important roles in the regulation of blood glucose levels, in addition to the well-established role of the pancreas.
Professor Inga Prokopenko, Professor of e-One Health and Head of Statistical Multi-Omics from the University of Surrey, said, “This hugely important study, involving over a hundred scientists from around the globe, gives us new insights into the genetics of blood glucose levels and type 2 diabetes. Already now, we can act on better prevention from type 2 diabetes complications, including lung disease. We should improve treatment strategies for people with this condition, by studying individual DNA variants in relation to GLP-1R agonist response.”
Individual genetic background affects GLP-1R agonist susceptibility.
GLP-1R agonists are used to treat individuals with type 2 diabetes to improve their glycemic control, meaning their ability to keep blood sugar levels within a normal range. Additionally, GLP-1R agonists have become very popular in helping individuals with type 2 diabetes lose weight, which can also improve their health and quality of life.
The scientists conducted functional and structural characterization of coding DNA variants in the GLP1R (GLP-1 receptor) gene. The authors highlight that the effect on GLP-1 receptor function and response to GLP-1R agonist drugs might differ from one individual to another depending on the GLP1R gene coding DNA variants they carry.
Overall, patients carrying specific DNA variants in the GLP1R gene are less likely to benefit from GLP-1R agonist drug treatment. The researchers suggest that doctors should pay more attention to individuals who get prescribed these specific drugs. By better matching the drug to the person’s unique makeup, the treatment is more likely to work well for them.
Type 2 diabetes causes reduced lung function.
Previous studies have shown that lung conditions, including restrictive lung disease, fibrosis, and pneumonia, are more common in people with type 2 diabetes. However, until now, it was not known whether type 2 diabetes directly causes damage to the lungs or if other factors common to both conditions are responsible.
Controlling for factors including smoking and sedentary behavior, the team used a statistical technique called Mendelian randomization to understand whether high blood sugar levels were linked to impaired lung function and whether one was causing the other. Lung function was measured using two common spirometry tests.
The analysis revealed that high blood sugar levels in people with type 2 diabetes directly impaired lung function. For example, modeling of the study data showed that an increase in average blood sugar levels from 4 mmol/L to 12 mmol/L could result in a 20% drop in lung capacity and function.
With respiratory disease being the third biggest cause of death in England and hospital admissions for respiratory diseases in England and Wales having doubled over the last 20 years, the findings highlight the need for health care professionals to be vigilant about lung complications in people with type 2 diabetes. Diagnosing and treating lung disorders early could potentially save the lives of thousands of people with type 2 diabetes.
Role of gastrointestinal tract in glucose levels regulation
The study identified roles for tissues not previously implicated in glucose metabolism, most notably those belonging to the intestinal tract, specifically the ileum and colon. It is common knowledge that food eaten is received by the duodenum (the first part of the small intestine) and mixed there with digestive juices from the pancreas, liver, and gallbladder. The jejunum and ileum further break down food and absorb nutrients into the bloodstream.
The large intestine, also known as the colon, absorbs water and electrolytes from undigested food and hosts a diverse community of bacteria known as the gut microbiome. This study showed that the human gut microbiome and glycome are related to glucose levels regulation and highlighted the role of lactose- and galactose-based production of glucose by microbiome species Collinsella and Lachnospiraceae-FCS020.
Dr. Vasiliki Lagou, postdoctoral scientist from the Section of Statistical Multiomics, the first author on the paper, added, “Our research provides the first evidence that high blood sugar levels in type 2 diabetes can directly lead to lung damage. We hope our discovery that impaired lung function is a complication of type 2 diabetes is the first step towards increased awareness among health care professionals, leading to earlier diagnosis and treatment of lung conditions.”
Dr. Ayse Demirkan, Senior Lecturer in AI Multiomics for Health and Wellbeing from the University of Surrey, commented, “Our study illuminates a less studied but hugely impactful role of the gastrointestinal tract in the regulation of blood sugar levels and type 2 diabetes. In addition to the pancreas, the small intestine and more specifically, the ileum, as well as the colon, contribute to glucose metabolism, as revealed by gene expression in these tissues.”
“Additionally, we report relationships between glucose level regulation and gut microbiome species, specifically Collinsella and Lachnospiraceae-FCS020, which produce glucose from lactose and galactose.”
More information:
Vasiliki Lagou et al, GWAS of random glucose in 476,326 individuals provide insights into diabetes pathophysiology, complications and treatment stratification, Nature Genetics (2023). DOI: 10.1038/s41588-023-01462-3. www.nature.com/articles/s41588-023-01462-3
Journal information:
Nature Genetics
Source: Read Full Article





