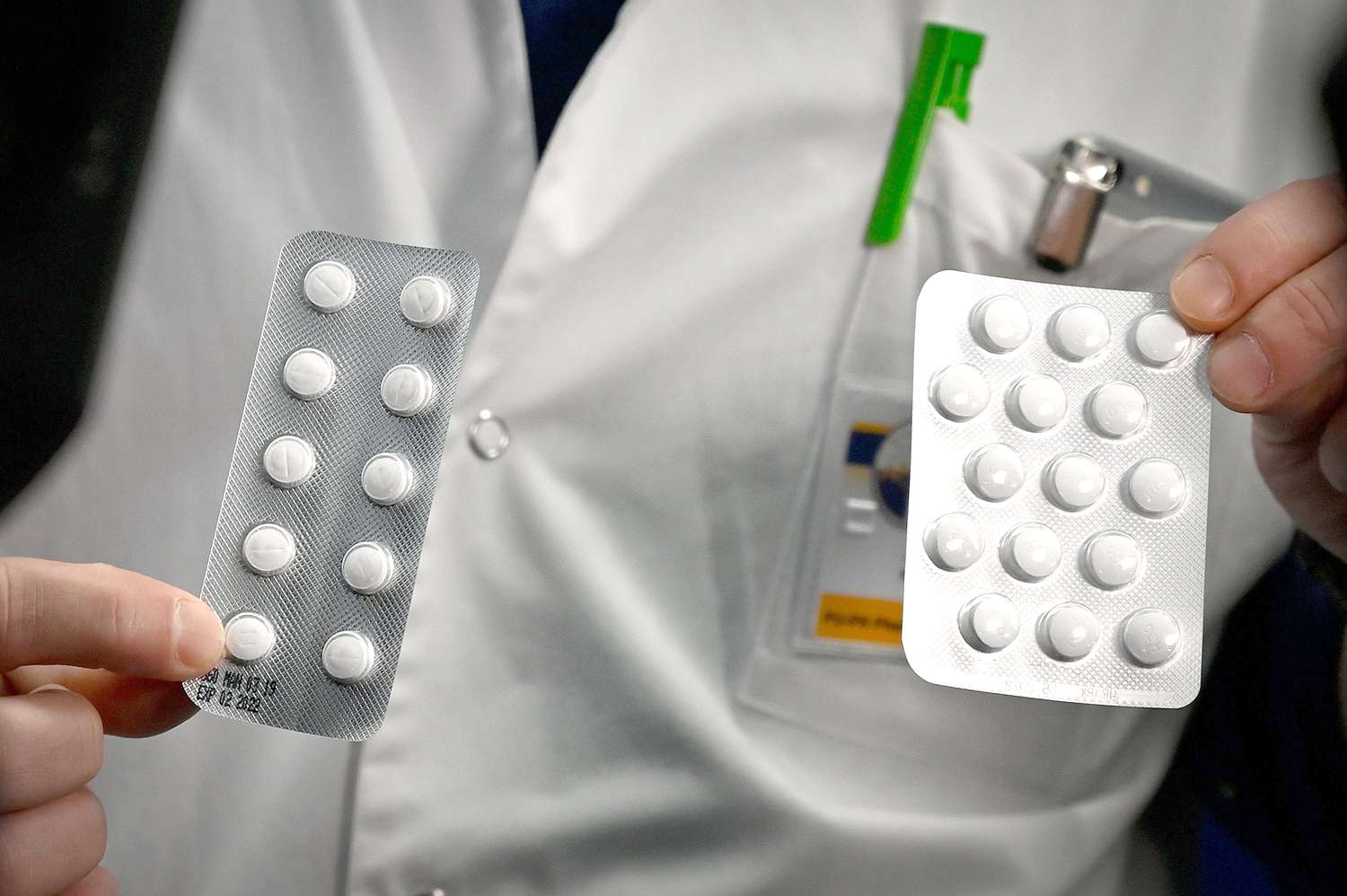
The anti-malarial drug hydroxychloroquine was ineffective when used on patients with the new coronavirus, COVID-19, and linked to a higher death rate, a new study found.
The drug was repeatedly touted by President Donald Trump as a COVID-19 treatment in mid-March, but health experts, including Food and Drug Administrator Stephen Hahn, emphasized that it needed to undergo trials to see if it would work against the virus. The FDA later granted emergency authorization to hospitals to use the drug in limited cases as a last-ditch treatment.
Several small studies on hydroxychloroquine have since come out, with poor results, particularly when the drug is used in combination with the antibiotic azithromycin. And now, a larger study on COVID-19 patients from Veterans Affairs came to a similar conclusion.
The VA study tracked 368 male COVID-19 patients, 97 of whom were given hydroxychloroquine and 113 of whom took hydroxychloroquine and azithromycin. The other 158 patients did not receive any hydroxychloroquine.
The patients who took the drugs died at a higher rate than those who did not. More than 27 percent of the hydroxychloroquine patients died, and 22 percent of those who took both hydroxychloroquine and azithromycin died. In contrast, those who did not take hydroxychloroquine had a rate of death more than two times lower, 11.4 percent.
More research into the drug is still needed, the study authors said, but their results indicate that hydroxychloroquine is not an effective treatment and should not be used on COVID-19 patients.
“An association of increased overall mortality was identified in patients treated with hydroxychloroquine alone,” the authors wrote. “These findings highlight the importance of awaiting the results of ongoing prospective, randomized, controlled studies before widespread adoption of these drugs.”
There are still many unknowns about COVID-19, including how best to treat the virus. There are other, ongoing studies into the effectiveness of hydroxychloroquine, along with remdesivir, an experimental antiviral drug. Researchers and doctors are also testing out treating COVID-19 patients with plasma from people who have already recovered from the virus.
But all need further testing before they’re put to use.
“Until we have good, solid evidence that something works, we shouldn’t use it [widely],” Dr. Emily Gurley, an infectious disease epidemiologist at the Johns Hopkins Bloomberg School of Public Health, previously told PEOPLE.
As information about the coronavirus pandemic rapidly changes, PEOPLE is committed to providing the most recent data in our coverage. Some of the information in this story may have changed after publication. For the latest on COVID-19, readers are encouraged to use online resources from CDC, WHO, and local public health departments. PEOPLE has partnered with GoFundMe to raise money for the COVID-19 Relief Fund, a GoFundMe.org fundraiser to support everything from frontline responders to families in need, as well as organizations helping communities. For more information or to donate, click here.
Source: Read Full Article