University of California, Irvine neuroscientists probing the gene changes behind Alzheimer’s disease have developed a process of making a “meta-cell” that overcomes the challenges of studying a single cell. Their technique has already revealed important new information and can be used to study other diseases throughout the body. Details about the meta-cell—created by researchers with the UCI Institute for Memory Impairments and Neurological Disorders, known as UCI MIND—are published in the online journal Cell Reports Methods.
Technologies called transcriptomics that study sets of RNA within organisms enable scientists to understand what each cell does. However, the question of how particular genes work within a solo cell, a process known as single-cell genomics, has not been widely studied. As a result, it has still been difficult to determine which genes are associated with disease or carrying out normal functions.
“The challenge is that a single cell does not contain much RNA,” said first author Samuel Morabito, a UCI graduate student researcher in the mathematical, computational and systems biology program. “This sparsity makes it hard to study. Even if a gene is present, technology might miss it.”
However, single-cell genomics is a powerful tool in the search for disease prevention and cures.
“If we know that a gene process is degrading cells, we can potentially intervene,” said lead author Vivek Swarup, UCI assistant professor of neurobiology and behavior. “We can devise therapeutics and target hundreds of genes to stop disease from developing.”
The team has now devised a way to eliminate this obstacle. “In working with an individual cell, we looked for others that are the most similar in terms of transcriptomics,” Swarup said. “By taking an average of 50 such cells, we developed a meta-cell that represents an individual cell but without the scarcity problems.”
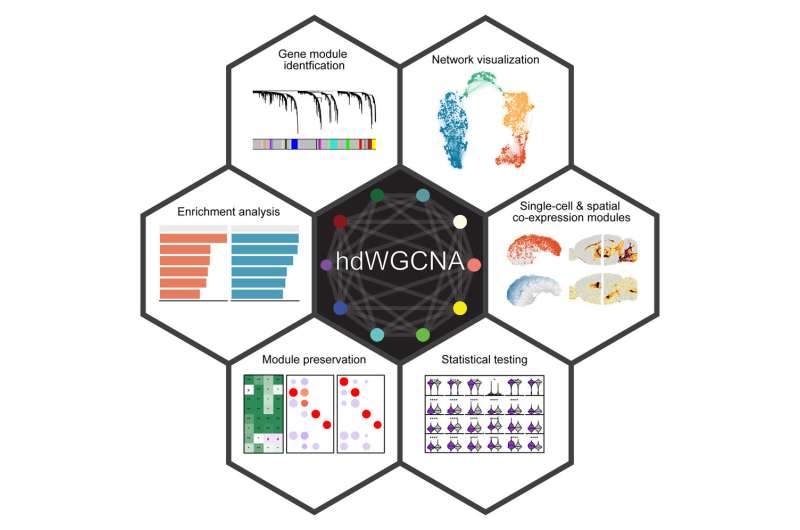
The new process, named hdWGCNA, improves on a method called RNA bulk sequencing that’s widely used but does not address single-cell genomes. The researchers utilized their laboratory’s own data and information from two other published studies to devise the process. They examined microglia, the brain’s primary immune cells, which carry most of the common Alzheimer’s genetic risk factors. Their findings revealed important insights and key areas to investigate further.
“We found it’s not easy to distinguish between good microglia that are doing their normal job and bad microglia that damage neurons,” Swarup said. “Normal brains have good microglia, but a large proportion of microglia in people with Alzheimer’s is altered to be reactive microglia. Also, the bad kind specific to Alzheimer’s has different types, and we discovered microglia states that were not previously known.” The team plans to next look at how genes regulate microglia and whether gene activity can be moderated or stopped through therapeutics.
While the researchers are focusing on neurological disorders, hdWGCNA is a versatile computational approach that can identify patterns of gene expression and gene modules associated with specific diseases, regardless of the organ or tissue involved.
More information:
Samuel Morabito et al, hdWGCNA identifies co-expression networks in high-dimensional transcriptomics data, Cell Reports Methods (2023). DOI: 10.1016/j.crmeth.2023.100498
Journal information:
Cell Reports Methods
Source: Read Full Article