A lung transplant often remains the only option for many patients with end-stage lung disease, a condition that can be brought on by emphysema, pulmonary fibrosis, cystic fibrosis and other lung disorders. However, the risk of organ failure and death following a lung transplant is high, particularly compared with the outcomes of transplant patients who receive new hearts, kidneys or livers.
Often, the culprit is lung rejection, which occurs when cells in the patient’s immune system perceive the donor lung as a foreign threat. This condition generally is treated by giving patients medications to suppress the immune system.
However, one particularly deadly form of rejection — called antibody-mediated rejection — remains difficult to diagnose after a lung transplant and is mostly impervious to available treatments. This process has been observed in more than 10 percent of lung transplant recipients. It occurs when a type of white blood cell, called B cells, from the recipient produces antibodies against the donor lung.
Now, a study led by researchers at Washington University School of Medicine in St. Louis, has identified, in mice, a process that may prevent antibody-mediated rejection and lead to the development of therapies to treat this form of rejection.
The study is published Feb. 1 in The Journal of Clinical Investigation.
“Antibody-mediated rejection is a vexing problem that is increasingly being acknowledged as an important cause of lung failure after transplantation,” said the study’s senior author, Daniel Kreisel, MD, PhD, the surgical director of lung transplantation at the School of Medicine and Barnes-Jewish Hospital. “Unfortunately, this complication can be fast-moving and hard to detect before it’s too late. Even when we do recognize it, current treatments have had limited success. Lung transplant centers — not just ours, but across the world — are losing too many patients to the condition.”
More than 4,200 lung transplants were performed worldwide in 2016, according to the 2018 International Society of Heart and Lung Transplantation Registry Report. Five years after lung transplantation, about half of the transplanted lungs are still functioning, according to the U.S. Organ Procurement and Transplantation Network. However, this compares with five-year organ survival rates of about 70 to 80 percent for liver, heart and kidney transplants.
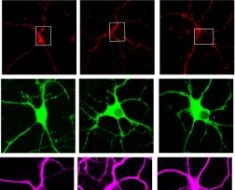
The study’s first author, WenJun Li, MD, an associate professor of surgery and director of microsurgery in the Thoracic Immunobiology Laboratory, developed a method for re-transplanting pea-sized mouse lungs. A re-transplant procedure involves the placement of a previously transplanted lung into a new host in order to assess immune networks that are established in the transplanted lung.
“This mouse lung re-transplant model is extremely delicate and technically challenging,” Li said. Using genetic tools, Li manipulated interactions involving immune cells within re-transplanted lung grafts.
The researchers found that giving mice immunosuppressive drugs at the time of transplant helped the lungs to survive and induced the growth of lymph node-like structures within the lung grafts. They discovered that these newly induced structures contained Foxp3-expressing T cells, a cell population that can dampen immune responses. The researchers noted that antibody-mediated rejection after lung transplantation occurs when B cells mingle with T cells in the donor lung. This co-mingling between B and T cells is prevented by Foxp3-expressing T cells. This suggests a treatment may be developed that interrupts the interaction so the two cell types cannot find each other.
“We thought these Foxp3 T-cells would inhibit activation of T cells after lung transplantation and prevent cellular rejection,” said Kreisel, who is also the G. Alexander Patterson, MD/Mid-America Transplant Endowed Distinguished Chair in Lung Transplantation. “Instead, we were surprised to find that it was the antibody-producing B cells that sparked lung rejection when Foxp3 T cells were absent.”
“Future research needs to show whether these findings will translate into human lung transplant recipients, and possibly to other organ transplant patients who develop antibody-mediated rejection,” Kreisel said. “The ultimate goal is to extend the lives of lung transplant recipients.”
Other institutions participating in the study include Northwestern University, the University of Virginia, the Cleveland Clinic and Duke University.
Source: Read Full Article