Neurodegenerative disorders like Alzheimer’s and Parkinson’s disease result from the loss of specific types of neurons due to abnormal accumulation of mutant proteins. Although specific brain regions have been found to be particularly vulnerable in each of these disorders, the causes and exact mechanisms underlying this differential vulnerability of brain cells and regions to toxic mutant proteins are not well understood.
A recent study from the lab of Dr. Huda Zoghbi, distinguished service professor at Baylor College of Medicine and founding director of the Jan and Dan Duncan Neurological Research Institute (Duncan NRI) at Texas Children’s Hospital, addressed this question in the context of spinocerebellar ataxia type 1 (SCA 1) and uncovered the diversity of molecular players and pathways that contribute to this neurodegenerative disorder.
The discovery, published in Neuron, charts an investigative path for a better understanding of regional vulnerability in other neurodegenerative disorders.
SCA1 is a progressive neurodegenerative disorder that affects about one or two in 100,000 people worldwide and is primarily characterized by loss of motor coordination and balance. It is caused by the presence of a mutant ATXN1 protein having long uninterrupted stretches called polyglutamine (polyQ) repeats.
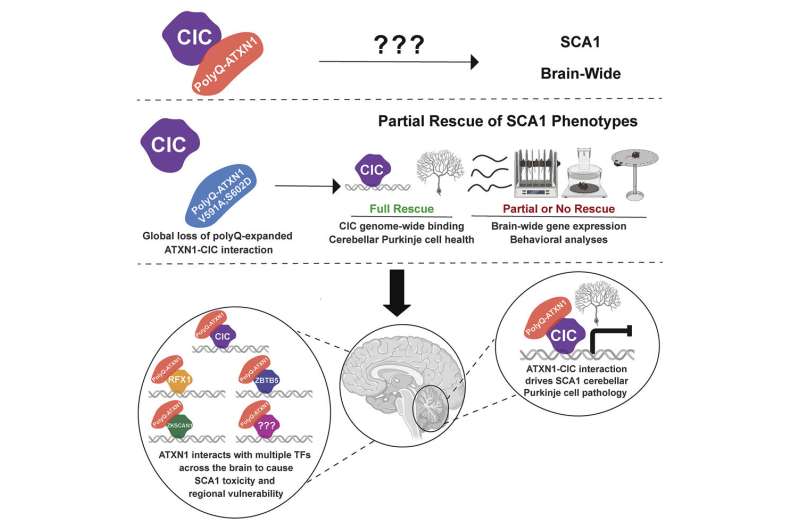
In healthy neurons, ATXN1 interacts with capicua (CIC), a protein that represses the expression of several genes. On the other hand, when mutant ATXN1 binds to CIC, CIC target genes are hyperexpressed, which is eventually toxic to neurons. Prior research has demonstrated that all changes in the cerebellar Purkinje cells in SCA1 mouse models result from the enhanced activity of the ATXN1-CIC complex.
However, ATXN1 protein is widely expressed and functions in various regions of the brain.
“A key question that has surprisingly remained unexplored is whether CIC drives ATXN1’s toxicity in other brain regions or whether other molecular players drive toxicity in other affected neurons,” said Zoghbi, who also is a Howard Hughes Medical Institute investigator.
To address this question, Stephanie Coffin, a graduate student in the Zoghbi lab generated a new SCA1 mouse model to mimic the human ATXN1 mutation and its effect throughout the brain. In this model, the ATXN1 gene has an expanded polyglutamine stretch, but she also mutated the two amino acids that are critical for the ATXN1-CIC interaction.
The Zoghbi team found that these two mutations fully ablated the ATXN1-CIC interaction in all brain regions.
“Disruption of the ATXN1-CIC complex led to partial improvements in SCA1 neurological symptoms like motor incoordination, respiration and short lifespan and only brought back to normal a subset of the gene expression changes,” Coffin said.
“Together, these findings gave us a clue that CIC is likely not the sole ATXN1 interactor that is driving SCA1 and that additional molecular players may be contributing to the development and progression of the variety of neurological symptoms seen in SCA1 patients and animal models.”
To identify additional interactors of ATXN1, they performed an unbiased proteomics screen using immunoprecipitation and mass spectrometry.
“Given that transcriptional changes are a hallmark of SCA1, we focused on three transcriptional factors that are expressed in various regions of the human and mouse brains, called ZKSCAN1, ZBTB5 and RFX1,” added Coffin, who is currently a neuroscience program manager at Pelagos Pharma.
“We validated the interaction between ATXN1 and these newly identified partners and discovered that the expression of the genes regulated by RFX1 and ZKSCAN1 was altered in SCA1 mice and human neurons. Moreover, these two newly identified partners—RFX1 and ZKSCAN1—together with CIC are predicted to regulate about 33% of the genes whose expression is altered in SCA1 mouse models, highlighting the important role they play in the pathogenesis of this disorder.”
“We were quite surprised to discover that for this single gene disorder the mutant protein uses distinct partners to drive toxicity in different brain cells,” Zoghbi said. “In fact, this study underscores the importance of investigating partners of other disease-driving proteins not only for other polyglutamine diseases but, broadly, for all neurodegenerative disorders. It is only through such systematic studies that we can understand the mechanisms driving these diseases and be in a better position to explore therapeutic interventions.”
More information:
Stephanie L. Coffin et al, Disruption of the ATXN1-CIC complex reveals the role of additional nuclear ATXN1 interactors in spinocerebellar ataxia type 1, Neuron (2022). DOI: 10.1016/j.neuron.2022.11.016
Journal information:
Neuron
Source: Read Full Article