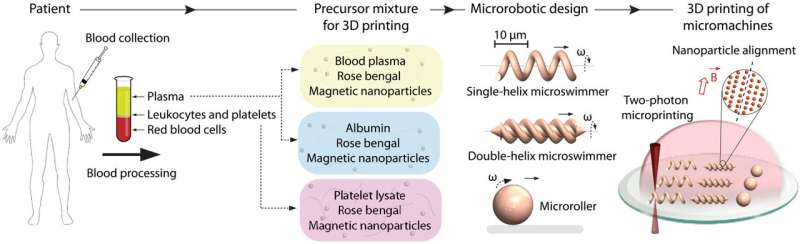
Wireless micromachines have increased the scope for applications in biomedicine, although their risks relative to minimal biocompatibility need be lessened. Most materials are not intrinsically biocompatible in physiological environments. In a new report now published in Science Advances, Hakan Ceylan and an international research team in physical intelligence, biomedical engineering and medicine have proposed a personalized approach for patient blood-derived biomaterials as a fundamental construction fabric to influence biocompatibility. Ceylan et al. developed the 3D printed multiresponsive microswimmers and microrollers using magnetic nanocomposites of blood plasma, serum albumin protein and platelet lysate that responded to time-variant magnetic fields for controlled cargo delivery and release. The proteinaceous fabrics also allowed enzymatic degradability to lower the risks of long-term toxicity. The resulting product can influence the development of many future medical robots and devices made of autologous biomaterials to improve biocompatibility and smart functionality in biomedical applications.
Wireless micromachines
Mobile and wireless micromachines are minimally invasive for targeted therapies in medicine. Despite their advanced design, fabrication and remote control, their interaction dynamics are usually unknown. As a result, deploying such micromachines in the body for long periods of time can pose substantial safety risks due to counteracting efforts to eliminate the product. Elimination depends on the construction material, time of exposure and the engineering design, which can activate defense cascades in vivo. To minimize the risk of cytotoxicity and the immune response, Ceylan et al. proposed a personalized approach with patient blood-derived biomacromolecules to form biocompatible micromachines. The personalized strategy for micromachine development can influence the design of a variety of medical robots and devices in the future for enhanced biocompatibility and intelligent functionality.
Concept and fabrication process
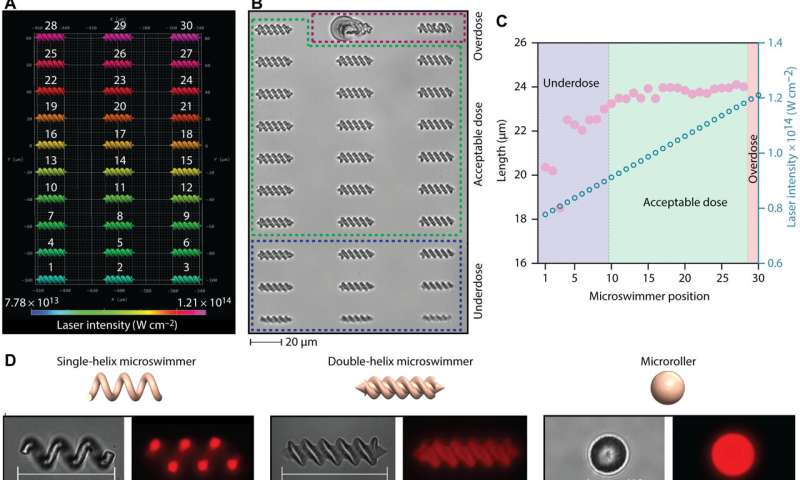
During the experiments, Ceylan et al. focused on blood components such as plasma, albumin and platelet lysate to develop the micromachines. Using two-photon polymerization based three-dimensional (3D) printing, the team fabricated a variety of medical micromachines with intricate features. The methods allowed highly complex 3D computer-aided designs with sub-micron features. The scientists developed micromachines from blood-harvested materials by creating a precursor mixture of rose Bengal and magnetic iron oxide nanoparticles. They then applied the fabrication approach on common and promising medical micromachine designs, including single helix microswimmers and microrollers at the microscopic length scale. The team optimized the laser intensity during 3D printing and developed a double-helix microswimmer array with varying laser intensities, and then viewed them under fluorescence microscopy to observe the high-fidelity printing process.
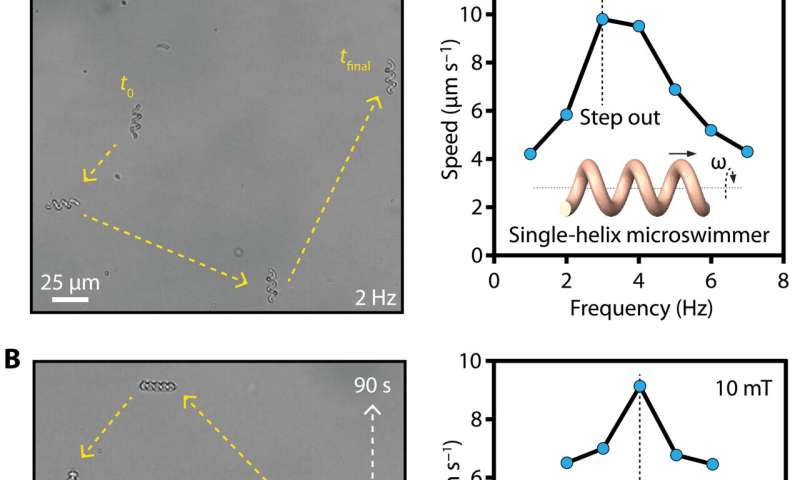
Magnetic actuation to steer the micromachines and properties of biodegradability
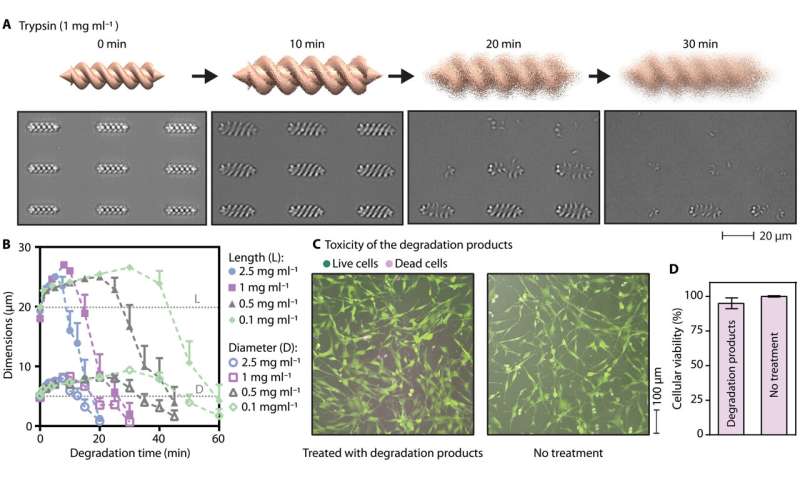
Ceylan et al. used rotational magnetic fields with a custom designed Helmholtz coil electromagnetic system mounted on an inverted microscope and observed how the single- and double-helix microstructures converted rotational motion into translational motion based on their asymmetric body shape. Meanwhile, microrollers converted the rotation into directional mobility based on nonslip contact with the surface. By increasing the magnetic nanoparticles loaded into the micromachines, Ceylan et al. facilitated fast locomotion of the micromachines. In addition to that, biodegradability of the medical micromachines also formed an essential aspect for long-term biocompatibility of the devices. For example, after explanting the micromachines, they should ideally dissolve in nontoxic soluble compounds, since an extended presence of nondegradable micromachines can result in chronic inflammation. To accomplish this, Ceylan et al. used trypsin as a model protease, or degrading enzyme, to understand the enzymatic degradability of micromachines. The scientists noted the degradation mechanisms and observed how the albumin microswimmers underwent rapid swelling followed by complete dissolution of their constituent hydrogel network under a variety of trypsin concentrations.
Cytotoxicity and pH-responsive shape memory behavior
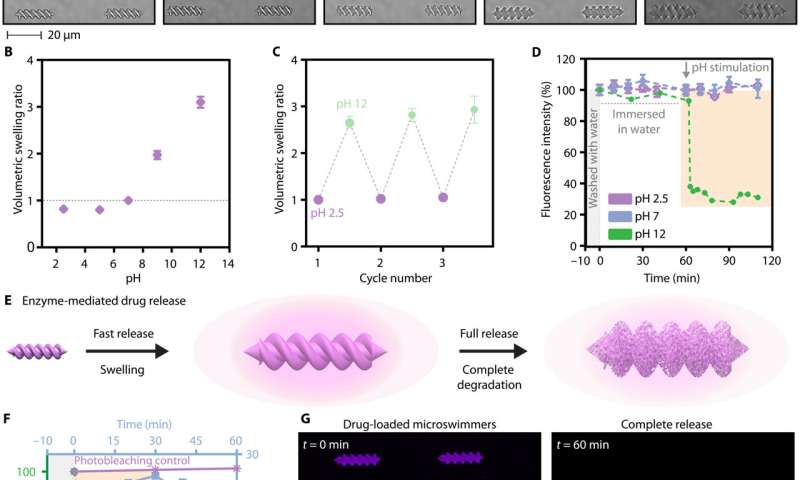
The team further studied the biocompatibility of the micromachines by exposing cells to albumin microswimmers and to enzymatic degradation. The cytotoxicity tests detected the cellular membrane integrity and metabolic activity to indicate how the sensitive stem cells did not pose an acute toxic response. Since proteins also contain a variety of amino acid and carboxylic acid groups that can dynamically change the protonation state relative to the environmental pH, Ceylan et al. investigated albumin and platelet lysate microswimmers in the pH ranges of 2.5 to 12 and noted how the albumin microswimmers demonstrated shape memory behavior when the pH reverted to its original value. The products showed two-way shape memory behavior for stability and robustness in response to physiologically relevant extreme pH changes that are likely to occur in microenvironments.
Stimuli-responsive cargo release
Drug delivery applications of medical micromachines also require on-demand and on-target therapeutic release. For instance, smart material systems with responsiveness to external triggers and environmental changes can improve controlled cargo release. As a result, the pH and enzyme sensitive protein-based microswimmers served as environmentally responsive drug delivery and release platforms. As proof of concept, Ceylan et al. loaded a fluorescent small molecule drug analog and a deep red dye into a porous network of the albumin microswimmers and then tested the release of the molecule by probing the fluorescence intensity in the microswimmer relative to pH changes and enzymatic degradation in the physiological microenvironment. The team identified the inherent responsiveness to pH and proteases to tailor personalized micromachines for specific biomedical applications. The personalized robotic materials developed in this manner can affect the design of many medical robots and devices with improved biocompatibility.
Outlook
Source: Read Full Article