By monitoring early-response biomarkers in men undergoing 177Lu-PSMA prostate cancer treatment, physicians can personalize dosing intervals, significantly improving patient outcomes.
In a study presented at the Society of Nuclear Medicine and Molecular Imaging 2023 Annual Meeting, early stratification with 177Lu-SPECT/CT allowed men responding to treatment to take a “treatment holiday” and allowed those not responding the option to switch to another treatment. The research was also published in the Journal of Nuclear Medicine.
Approved by the U.S. Food and Drug Administration in 2022, 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer. However, not all men respond equally to treatment, with some responding very well and others progressing early.
“Currently, a standardized dosing interval is used for 177Lu-PSMA treatment,” said Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the Department of Theranostics and Nuclear Medicine at St. Vincent’s Hospital in Sydney, Australia. “However, monitoring early-response biomarkers to adjust treatment intervals may improve patient outcomes.”
In the study, researchers sought to evaluate progression-free survival and overall survival of different dosing intervals. Study participants included 125 men who were treated in a clinical program with six weekly doses of 177Lu-PSMA. The men were imaged with 177Lu-SPECT/CT after each dose. After the second dose, researchers analyzed the men’s prostate specific antigen (PSA) levels and the 177Lu-SPECT response to determine ongoing management.
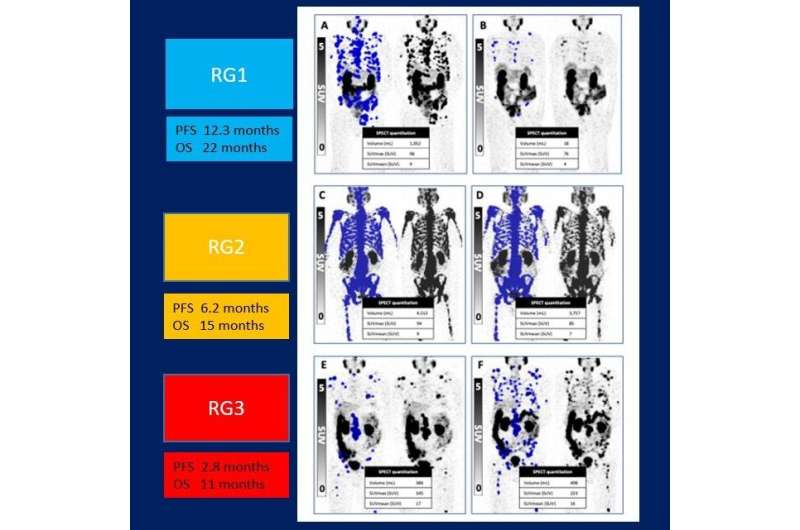
Patients were grouped by level of response. Those in Response Group 1 (35% of participants) had a marked reduction in PSA level and partial response on 177Lu-SPECT and were advised to cease treatment until PSA levels rose. Response Group 2 (34%) saw stable or reduced PSA and stable disease on SPECT imaging; these men continued on their six week treatment plan until no longer clinically beneficial. In Response Group 3 (31%), men saw a rise in PSA levels and had progressive disease on SPECT imaging. These patients were offered the opportunity to try a different treatment.
PSA levels decreased by more than 50% in 60% of patients. Overall study participants had a median PSA progression-free survival of 6.1 months and a median overall survival of 16.8 months. Median PSA progression-free survival was 12.1 months, 6.1 months, and 2.6 months, for Response Groups 1, 2 and 3, respectively. The overall survival was 19.2 months for Response Group 1, 13.2 months for Response Group 2, and 11. 2 months for Response Group 3. Additionally, for those in Response Group 1 who had a “treatment holiday,” the median treatment-free time was 6.1 months.
“Personalized dosing allowed one-third of the men in this study to have treatment breaks while still achieving the same progression-free and overall survival outcomes they would have if they received continuous treatment,” noted Nguyen. “It also allowed another one-third of men who had early biomarkers of disease progression the opportunity to try a more effective potential therapy if one was available.”
Patients at St. Vincent’s Hospital will continue to be stratified by these early response biomarkers. Once validated in a prospective clinical trial, Nguyen hopes that this stratification strategy will become more widely available for patients.
More information:
Patient outcomes following a response biomarker guided approach to treatment using 177Lu-PSMA-I&T in men with metastatic castrate resistant prostate cancer (Re-SPECT), Journal of Nuclear Medicine (2023).
Journal information:
Journal of Nuclear Medicine
Source: Read Full Article