After 30 years of discouraging results in attempting to develop drugs to inhibit a mutated protein associated with some of the more challenging cancers to treat, research on RAS proteins is booming.
New discoveries have revised the notion that RAS is an “undruggable” target or that individual RAS mutations are indistinguishable in their effects, said MUSC Hollings Cancer Center researcher John O’Bryan, Ph.D.
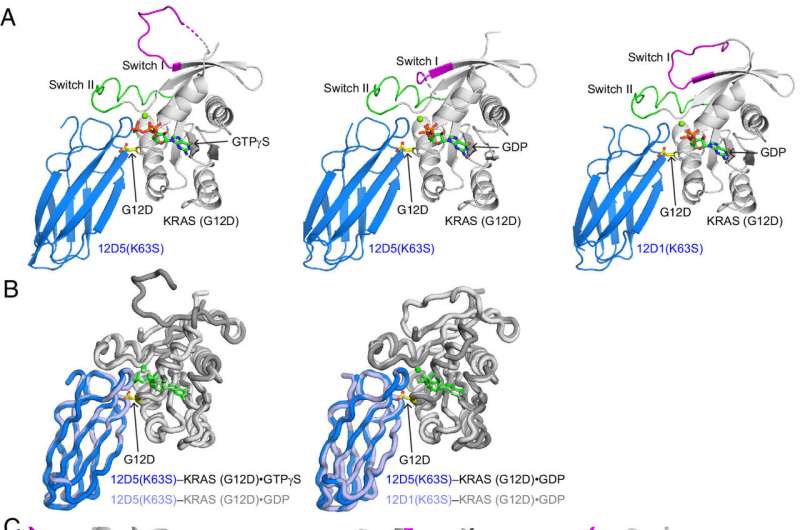
O’Bryan, along with his longtime research partner Shohei Koide, Ph.D., director of Cancer Biologics at Perlmutter Cancer Center at NYU Langone, and additional collaborators at Hollings and Perlmutter, have now added to this growing body of knowledge with their development of synthetic monobodies that not only attach to KRAS(G12D), a specific RAS mutation that is common in pancreatic, lung and colorectal cancers, but also inhibit some of KRAS(G12D)’s actions.
Crucially, the method that they used to develop the monobodies provides a blueprint for targeting other mutations currently considered “undruggable.”
Targeting KRAS
RAS proteins are biochemical on/off switches that regulate signaling for many growth factor and hormone receptors.
“It’s a really critical signal relay in the cell,” O’Bryan said. But mutations mean that RAS gets stuck in the “on” position, leading to uncontrolled growth and, eventually, cancer.
O’Bryan and Koide have been working together on RAS for more than a decade. In their paper published in the Proceedings of the National Academy of Sciences they report on these synthetic monobodies as well as the structure of a hidden pocket on KRAS(G12D) that researchers are targeting as a secret entrance for cancer-fighting drugs.
“When you make a monobody, you don’t know what it’s going to do,” O’Bryan explained. “It may just bind and not have any effect. But it turns out that almost all of the monobodies we’ve made to RAS are inhibitory, meaning they bind to important regions that are necessary for the function of RAS. And so, by first demonstrating that they selectively bind and inhibit RAS, and then determining where they’re binding, we can gain insight into how those regions of RAS are important for the function of the protein.”
RAS mutations are present in about 20% of all human cancers, O’Bryan said, but can be found in upward of 90% of pancreatic ductal adenocarcinomas (PDAC), the most common type of pancreatic cancer.
“RAS is actually a driver of that tumor. It’s one of the initiating events of PDAC formation,” O’Bryan said.
Their publication comes at a time when the understanding of RAS is leaping forward. In the past two years, the Food and Drug Administration has approved two lung cancer drugs that target KRAS(G12C), another frequent RAS mutant protein. And this year, an early-stage clinical trial of a drug targeting KRAS(G12D) began. That drug’s development was only possible because of the work of Kevan Shokat, Ph.D., who uncovered the hidden pocket that these drugs target, O’Bryan said.
O’Bryan and Koide, however, began their work before anyone knew about that hidden pocket, by sorting through libraries of monobodies.
“It turns out our monobody binds around that pocket and actually opens it up more,” O’Bryan said. “That suggests that there may be some ways of using that information in drug design and development.”
In their paper, the researchers also describe how that hidden pocket is structured, which they believe provides important data points for those developing next-generation KRAS(G12D) inhibitors.
They also believe that the protein-engineering technologies used to develop their monobodies could be used against other challenging targets and ultimately prove a more straightforward approach.
Even as O’Bryan and Koide were polishing their paper for PNAS, their work on RAS continued, both individually and together.
O’Bryan was recently awarded a grant by the Department of Defense to work on delivering monobodies into the lungs as potential therapies, and he is working with Aaron Hobbs, Ph.D., a fellow Hollings scientist, whose research focuses on KRAS(G12R) in pancreatic cancer.
More information:
Padma Akkapeddi et al, Exploring switch II pocket conformation of KRAS(G12D) with mutant-selective monobody inhibitors, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2302485120
Journal information:
Proceedings of the National Academy of Sciences
Source: Read Full Article