Antibodies generated by COVID-19 vaccines are more suited to recognizing viral variants than antibodies that arise from natural infection, according to a study by researchers at Stanford Medicine.
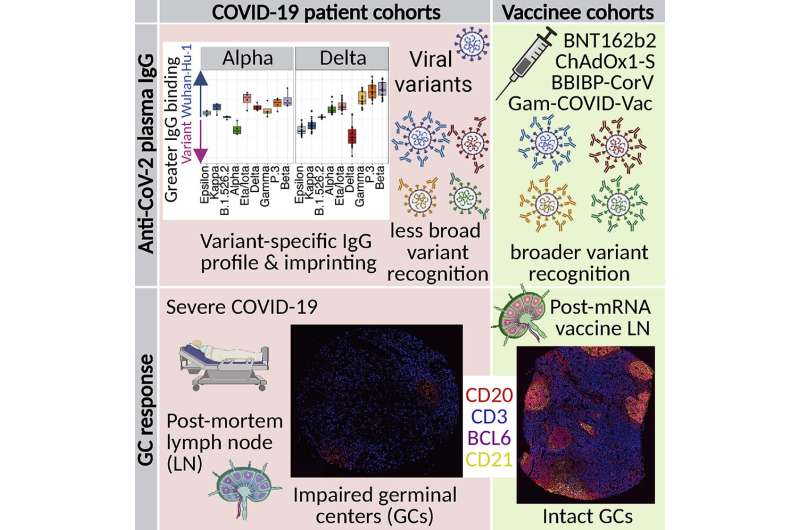
A key finding of the study might explain why: Regions in lymph nodes known as germinal centers—where antibodies are chosen and amplified by the immune system—are highly active for several weeks after vaccination. In contrast, the structure and cell composition of germinal centers are profoundly disrupted in people with fatal cases of COVID-19.
“Vaccination generates a range of antibodies capable of responding to viral antigens beyond the original exposure,” said Scott Boyd, MD, Ph.D., associate professor of pathology. “This greater breadth of antibodies suggests that vaccination is likely to be more protective against viral variants than immunity generated by previous infection.”
Boyd shares a senior authorship of the study, which was published online Jan. 24 in Cell, with Kari Nadeau, MD, Ph.D., the Naddisy Foundation Professor in Pediatric Food Allergy, Immunology, and Asthma. The lead authors are postdoctoral scholar Katharina Röltgen, Ph.D.; former postdoctoral scholar Sandra Nielsen, Ph.D.; clinical assistant professor of pathology Oscar Silva, MD, Ph.D.; and life science researcher Sheren Younes, MD, Ph.D.
Response biased toward first viral exposure
The study also showed for the first time that a person’s immune response to viral variants is strongly influenced by the version of the COVID-19 virus, known as SARS-CoV-2, first encountered by the immune system.
This phenomenon, known as imprinting, is similar to what is seen with influenza viruses that vary from season to season, and it creates a unique immune landscape for each individual. Prior exposures with different influenza viruses can either strengthen, or sometimes impair, responses to new influenza virus types. Understanding what imprinting means with regard to immunity against ever-evolving SARS-CoV-2 variants will be a focus of future investigation, and it could guide decisions about vaccine booster composition and timing, the scientists say.
The researchers compared the levels and types of antibodies to SARS-CoV-2 in participants in Stanford’s clinical trial of the BioNTech-Pfizer vaccine with those of COVID-19 patients treated at Stanford Medicine during the first months of the pandemic. These patients were treated before the widespread circulation of SARS-CoV-2 in the United States and were most likely infected with one of the earliest strains of the virus, known as Wuhan-Hu-1.
“When we compared antibody responses to infection with those from vaccination, we found that infected people generated variable levels of antibodies, which dropped steadily after infection,” Röltgen said. “In contrast, the response to vaccination was very uniform—all the study participants had a good response, with high levels of antibodies, although these also decreased over time.”
The mRNA-based vaccine generated the highest levels of antibodies in the researchers’ comparison of results from people studied at the Onom Foundation and Liver Center in Mongolia who had been vaccinated with one of three other globally available SARS-CoV-2 vaccines: the adenovirus-based vaccines produced by Cambridge-based AstraZeneca and the Russian vaccine Sputnik V, and an inactivated virus vaccine produced by the Chinese company Sinopharm. All of these vaccines work by exposing the immune systems to portions or all of the Wuhan-Hu-1 virus.
Vaccines generate broad range of antibodies
Each of the vaccines generated a broad range of antibodies. Although most antibodies targeted the viral spike protein of the Wuhan-Hu-1 virus, others could also bind to the spike proteins of nine other viral variants, including the delta variant that caused a surge in hospitalizations and deaths in the late summer and fall of 2021 in the United States. In contrast, unvaccinated patients infected with Wuhan-Hu-1 made a narrower range of antibodies, fewer of which could bind to the spike proteins of the variants.
“Our data indicate that infection with a particular viral variant gives an antibody response that is focused on the antigens from that variant, and it doesn’t have as much breadth of binding to different variants as the vaccine-induced antibody response,” Boyd said. “These results support and extend findings reported for certain viral mutations by previous studies.”
When the researchers examined the lymph nodes of vaccinated and infected people, they saw that the germinal centers in those who were vaccinated appeared to be potent antibody-generating factories for as long as eight weeks after vaccination. In contrast, the germinal centers of lymph nodes in people with severe COVID-19 were poorly formed and were missing key immune cell types, suggesting an impairment of their ability to make disease-fighting antibodies.
The researchers also studied vaccinated and unvaccinated people who became infected with the alpha or delta variants that succeeded the original viral strain. They found that unvaccinated people infected with alpha or delta made antibodies that are specialized in binding to the viral spike protein of alpha or delta, respectively. Previously vaccinated people who were subsequently infected with alpha or delta variant viruses made a panel of antibodies that recognized the spike protein from the Wuhan-Hu-1 strain, which was first presented to them in the vaccine, as well as those of other variants, including alpha and delta.
In previously vaccinated people, “there is less bias of the antibody response toward the variant that has infected you,” Boyd said. This could mean that vaccinated people are better prepared than naturally infected people to fend off subsequent infections with other variants.
It’s unclear whether booster shots tailored to emerging new variants will also be effective in eliciting immune responses to future variants. If a variant arises that is significantly different from the Wuhan-Hu-1 strain used to make the currently available vaccines—and from the variants that preceded it—it may be useful to prime our immune systems with the new information, the researchers said.
In any case, the current vaccines generate broad protection from severe disease caused by variants—one that surpasses the effect of infection.
Source: Read Full Article