Scientists affiliated with the Knight Initiative for Brain Resilience at Wu Tsai Neuro have discovered a surprising connection between brain cells involved in producing the insulation around nerve fibers, our sleep patterns, and neurodegenerative diseases such as multiple sclerosis (MS).
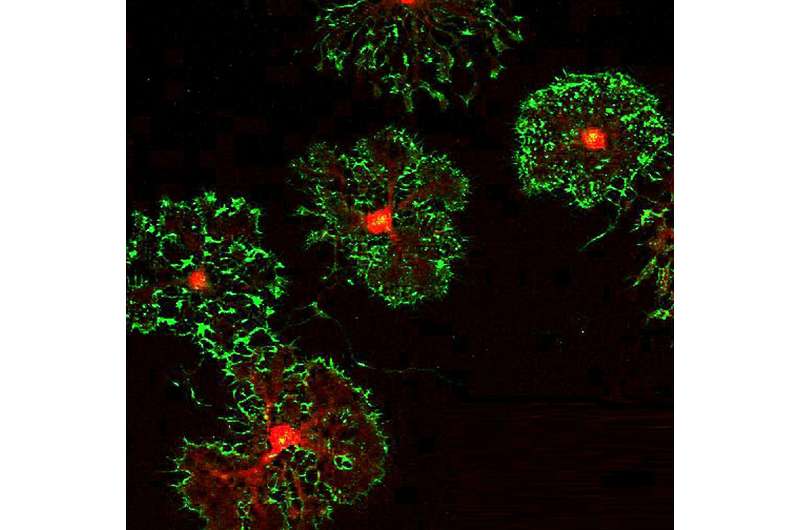
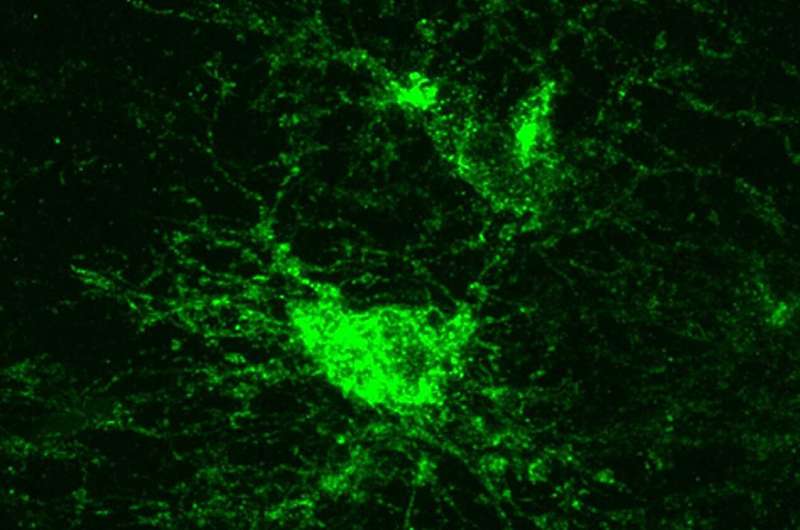
The cells studied are a type of glial cell known as oligodendrocyte precursor cells (OPCs). As the name implies, these cells can mature into oligodendrocytes, which are responsible for making myelin—the insulation that ensheaths nerve fibers throughout the brain and speeds neuronal signaling. But before OPCs turn into oligodendrocytes, they have a whole host of other duties, including playing a role in immune responses and even forming synapses with neurons.
“To me, OPCs are the most interesting cell in the central nervous system, partly because in many ways, they can act like all the other cells in the central nervous system,” said Erin Gibson, an assistant professor of psychiatry and behavioral sciences and lead author on the new work. The paper was published on August 31 in Neuron with postdoc Daniela Rojo, a Brain Resilience Scholar supported by the Knight Initiative, as first author.
Gibson, Rojo, and their colleagues revealed that another unexpected role of OPCs may involve sleep and have important implications for brain health, especially in night shift workers.
Circadian gene regulates brain insulation
As a circadian biologist, Gibson is familiar with the effects that our circadian system imparts on every bodily process. This system regulates activity to oscillate on a 24-hour rhythm, such that at night, organs like the stomach decrease their digestion and our overall body temperature drops. Gibson wondered if glial cells like OPCs might also have their own 24-hour circadian rhythms. The new research showed that indeed they do, with 10% of genes in OPCs oscillating over a 24-hour day, the first evidence that OPCs are strongly controlled by the circadian system.
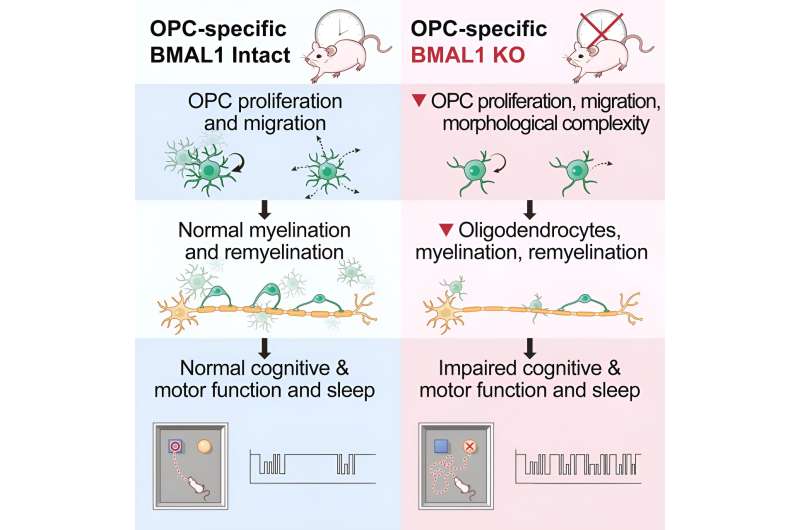
Gibson and Rojo’s team also revealed that when the circadian regulation of OPCs breaks down, an array of harmful effects ensue—impacting both sleep patterns and the health of the insulating myelin surrounding neurons. In OPCs that were missing a crucial aspect of the circadian regulation system—a gene called Bmal1—the researchers observed abnormal effects. These OPCs did not divide and produce other OPCs as frequently, nor did they migrate to areas of the brain where they were needed.
They even looked strange. “One of the most shocking things was how different these OPCs looked following Bmal1 removal,” said Gibson. “OPCs are normally these beautiful cells with complex tree-like morphology. But these cells were completely truncated. ”
These changes to the OPCs resulted in decreased myelin insulation and impaired myelin repair after an injury. The abnormalities were the worst for mice who had Bmal1 removed as embryos rather than in adolescence or adulthood, suggesting an important role of this circadian gene during brain development.
The impaired myelination also impacted animals’ cognition and movement—mice lacking Bmal1 showed deficits on an attention task and slower swinging of their paws while walking around.
Linking sleep disruption to cognitive decline
Of all the effects they studied, the team was most interested in the impact that dysregulated OPCs had on sleep. Specifically, the mice had a harder time remaining in a deep sleep or non-REM phase, which led to more frequent naps during their active phase when they should have been awake.
“What was interesting about that is that increases in so-called active phase napping is now believed to be one of the earliest signs of Alzheimer’s disease,” said Gibson.
The researchers were curious whether this type of sleep deficit, called sleep fragmentation, might also be found in other neurodegenerative diseases. They went searching for gene variants associated with sleep fragmentation in two large human genetic datasets and found the phenomenon was linked to a higher risk of developing MS.
It’s possible, the researchers realized, that the increased risk of MS—a demyelinating disease—could be related to the finding that mice with OPCs lacking the Bmal1 gene had more difficulty recovering myelin after a demyelinating injury.
“That was very surprising to me. I was not expecting such a result,” said Rojo. She adds that this discovery “can give us an idea of why some disruptions in circadian shifts in sleep, for example, in night workers, can increase their risk of having demyelinating disorders like multiple sclerosis.”
Treatments based on the clock
For now, it remains to be seen whether the abnormal myelination and sleep fragmentation found in mice was directly caused by the lack of the Bmal1 in the cells, or by its disruption to myelin, or even whether another gene besides Bmal1 could have similar effects. Gibson and Rojo’s team are now working on experiments to reveal how Bmal1 is regulating OPCs on a more granular level.
But if dysregulation of the OPCs by Bmal1 loss does cause similar effects in humans and contributes to diseases like MS, Gibson said that it could be used as a new therapeutic target.
“We haven’t really done a great job of developing drugs that target an enhancement in oligodendrogenesis and remyelination,” she said. “This tells us that this is a new molecular pathway that plays a role in how these OPCs proliferate and differentiate and migrate into lesions. And so if we can target it, maybe we might be able to make that process more efficient.”
Plus, Gibson adds that their new results could even mean that a so-called “chronotherapeutic” approach could be useful for diseases like MS that impact myelin, such that drugs that are currently on the market to target oligodendrocytes could be more effective if given at certain times of day.
Moving forward, Gibson plans to further investigate the sleep changes to reveal exactly what causes them and whether other types of myelin dysregulation also cause sleep issues.
“As a field, we’ve never looked into this relationship,” said Gibson. With further research to pin down how oligodendrocytes and myelin affect sleep in normal development and disease states, Gibson adds that “we might be able to understand this intersection of myelin and sleep biology.”
More information:
Daniela Rojo et al, BMAL1 loss in oligodendroglia contributes to abnormal myelination and sleep, Neuron (2023). DOI: 10.1016/j.neuron.2023.08.002
Journal information:
Neuron
Source: Read Full Article