Van Andel Institute scientists have pinpointed how a specific gene mutation triggers an inflammatory cascade that may drive development of treatment-resistant cancers.
The new findings, published today in Molecular Cell, reveal for the first time the molecular circuitry by which mutations in the gene STK11 cause inflammation to spiral out of control. The resulting chemical firestorm damages healthy cells and can enable cancer development. Tumors that lose the STK11 gene are tough to treat because they resist traditional chemotherapy and many of the latest immunotherapies.
“Understanding how and why this mutation leads to cancer is a critical step in developing improved treatments,” said Russell Jones, Ph.D., chair of VAI’s Department of Metabolism and Nutritional Programming and the study’s corresponding author. “Our study identifies important features of these cancers and suggests that targeting inflammation may make these tumors more responsive to treatment.”
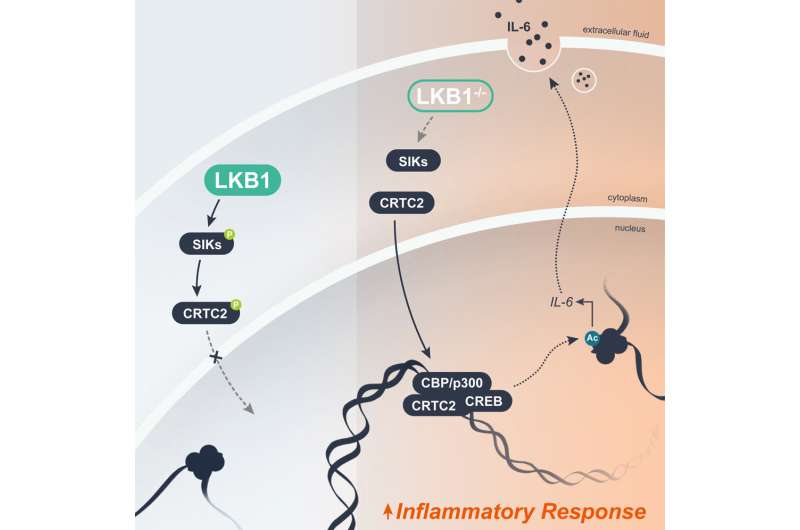
STK11 mutations occur when the instructions encoded by the gene are disrupted. This causes the gene to produce insufficient levels of the protein LKB1, a tumor suppressor that keeps cell growth under control. Loss of LKB1 derails critical cellular checks and balances and allows cancerous cells to grow unimpeded.
Loss of LKB1 is among the most common genetic mutations in human cancer, especially treatment-resistant lung cancer and pancreatic and cervical cancers. It also is the main player in the rare disease Peutz-Jehgers Syndrome. People with Peutz-Jehgers Syndrome develop polyps in their digestive tract and have a significant increase in cancer risk.
Although LKB1 has long been linked to cancer, it was not clear exactly how it worked to promote tumor growth. Earlier research by Jones and colleagues hinted that control of inflammation by LKB1 may be the culprit but the mechanism was unknown. Today’s findings offer a clearer picture of the process.
LKB1 works in part by regulating inflammation, which is a normal part of the body’s defense mechanisms against injury and infection. When LKB1 is lost, it triggers an epigenetic change in cells, which affects how the instructions encoded in DNA are acted upon. This results in rampant inflammation that damages healthy cells and may push them closer to malignancy.
“It’s a perfect storm of problems for which we now have potential solutions,” said Shelby Compton, a Van Andel Institute Graduate School Ph.D. student and the study’s first author. “In addition to cancer, I hope this work will inform new therapeutic strategies for Peutz-Jehgers Syndrome, which has few treatments and no cure.”
The next step, Jones says, is to “put ideas into action” by developing strategies to target inflammation in LKB1-associated cancers. The team also plans to continue exploring LKB1 in Peutz-Jehgers Syndrome with the goal of working toward new, much-needed therapies for people with this condition.
More information:
Shelby E. Compton et al, LKB1 controls inflammatory potential through CRTC2-dependent histone acetylation, Molecular Cell (2023). DOI: 10.1016/j.molcel.2023.04.017
Journal information:
Molecular Cell
Source: Read Full Article