Data from the phase III INTERACT3 study demonstrate that a new combination of treatments for stroke due to intracerebral hemorrhage (ICH) significantly improves the chances of surviving without major disability. Results were presented at the European Stroke Organization Conference in Munich, Germany, and published in The Lancet.
The INTERACT3 study is the first-ever randomized controlled trial to show a clearly positive outcome for the treatment of ICH. Timely administration of the new treatment protocol—known as a Care Bundle—centered on the rapid control of high blood pressure, led to improved recovery, lower rates of death, and better overall quality of life in patients with this serious condition.
Professor Craig Anderson, Director of Global Brain Health at The George Institute and a senior author of the research said, “Despite the high rates of ICH and its severity, there are few proven options for treating it, but early control of high blood pressure is the most promising. Time is critical when treating this type of stroke, so we tested a combination of interventions to rapidly stabilize the condition of these patients to improve their outcomes. We estimate that if this protocol was universally adopted, it could save tens of thousands of lives each year around the world.”
Commonly referred to as a hemorrhagic stroke or brain bleed, ICH is the second most common type of stroke and also the most deadly, with 40% to 50% of patients dying within 30 days. It occurs when blood leaks out of a blood vessel into the brain tissue and represents over a quarter of all cases of stroke, affecting approximately 3.4 million people a year.
In the INTERACT3 study, over 7,000 patients were enrolled across 144 hospitals in 10 countries—nine middle-income countries and one high-income country.
The research team evaluated the effectiveness of the new Care Bundle, which included early intensive lowering of systolic blood pressure, strict glucose control, fever treatment, and rapid reversal of abnormal anticoagulation.
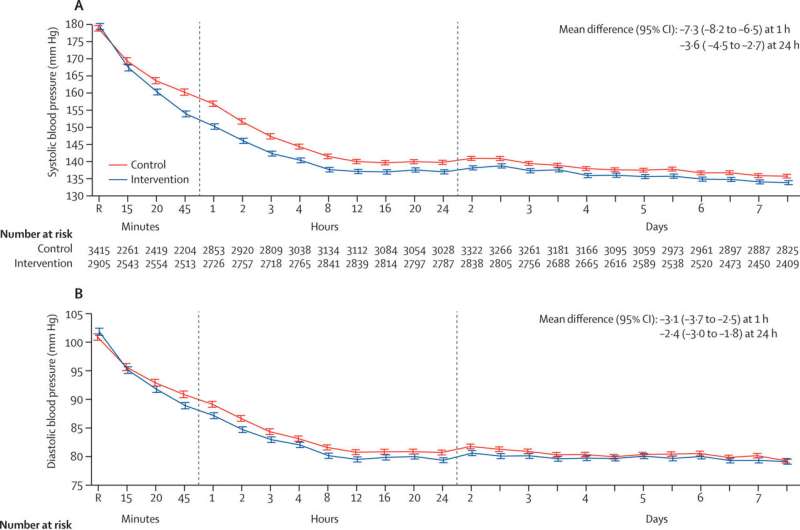
They found that using this new treatment protocol compared to usual care reduced the likelihood of a poor functional outcome, including death, after six months. This was estimated to prevent one additional death for every 35 patients treated.
Central to this was a rapid reduction in systolic blood pressure, where target levels were achieved, on average, in 2.3 hours (range 0.8 to 8.0hrs), compared to 4.0 hours (range 1.9 to 16.0hrs) in the control group. The interventional protocol resulted in a statistically significant reduction in mortality, number of serious adverse events, and time spent in hospital, as well as demonstrating an improvement in health-related quality of life.
The burden of ICH is greatest in low- and middle-income countries. In 2019, 30% of all stroke cases in LMICs were ICH, almost double the proportion seen in high-income countries (16%). This is in part due to high rates of hypertension and limited resources for primary prevention strategies, including identification and management of stroke risk factors by health care services.
Dr. Lili Song, joint lead author and Head of the Stroke Program at The George Institute China, said, “A lack of proven treatments for ICH has led to a pessimistic view that not much can be done for these patients. However, with INTERACT3, we demonstrate on a large scale how readily available treatments can be used to improve outcomes in resource-limited settings. We hope this evidence will inform clinical practice guidelines across the globe and help save many lives.”
More information:
The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial, The Lancet (2023). DOI: 10.1016/S0140-6736(23)00806-1. www.thelancet.com/journals/lan … (23)00806-1/fulltext
Journal information:
The Lancet
Source: Read Full Article