Estrogen receptor positive (ER+) breast cancer is the most common type of breast cancer, but resistance to therapy is common and eventual development of metastatic disease is a leading cause of death. In new research published in Cell Reports, researchers from Baylor College of Medicine and Washington University in St. Louis identify estrogen receptor alpha gene (ESR1) translocation events that drive not only therapeutic resistance, but also trigger ER+ breast cancer cells to metastasize.
“Breast cancer is not generally thought to be a disease driven by chromosomal translocations, when two separate genes break in two and then the ends find each other to create a chimera, or fusion, protein that is encoded by the front half of one gene and the back half of another,” said Jonathan Lei, graduate student in translational biology and molecular medicine at Baylor and first author on the paper. “However, we detected through RNA-sequencing the presence of ESR1 fusion transcripts in ER+ breast cancer, but we weren’t sure how they contributed to disease progression.”
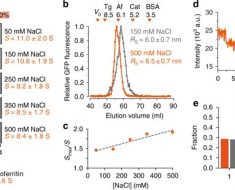
The research team studied ESR1 fusions identified in both treatment-naive primary breast tumors, or tumors that have not yet undergone treatment, as well as in late-stage, endocrine therapy-resistant, metastatic ER+ breast cancer patients. >From the metastatic patients, the researchers found two different ESR1 fusion events that generated very active estrogen receptor fusion proteins and went on to study their biological properties in detail.
These two hyperactive fusion proteins, part estrogen receptor and part fusion partner protein, caused profound endocrine therapy resistance because the part of the estrogen receptor that interacts with estrogen and with breast cancer drug tamoxifen was replaced with a protein fragment from the partner gene, which caused unregulated growth. More surprising was the ESR1 fusions also could promote cell motility, and cancer spread though the activation of a metastasis program called epithelial to mesenchymal transition. This potentially explains why these active ESR1 fusion genes have been found only in advanced breast cancer cases so far; they are the actual cause of metastasis.
The researchers also found a way to suppress ESR1 fusion-driven growth at primary and metastatic sites using existing FDA-approved breast cancer drugs that target CDK4/6 cell cycle proteins called palbiciclib and abemacicilib.
“These findings are important because they help explain how endocrine therapy drug resistance and metastasis are linked lethal processes. Our studies should drive more dedicated efforts to identify and characterize additional ESR1 fusions in early and late-stage ER+ breast cancer,” Lei added. More ESR1 fusions are being detected in metastatic breast cancer, and many precision medicine programs are now including RNA-sequencing in patient care plans as sequencing technologies continue to improve and become more cost effective.
“From the clinical perspective, this study suggests that the diagnosis of an active ESR1 fusion could guide treatment by selecting CDK4/6 inhibitor monotherapy for patients with highly endocrine therapy resistant metastatic ER positive disease where traditionally, chemotherapy has been the standard of care,” said Dr. Matthew Ellis, McNair Scholar and director of the Lester and Sue Smith Breast Center, part of the NCI-designated Dan L Duncan Comprehensive Cancer Center at Baylor, and senior author on the paper.
Source: Read Full Article