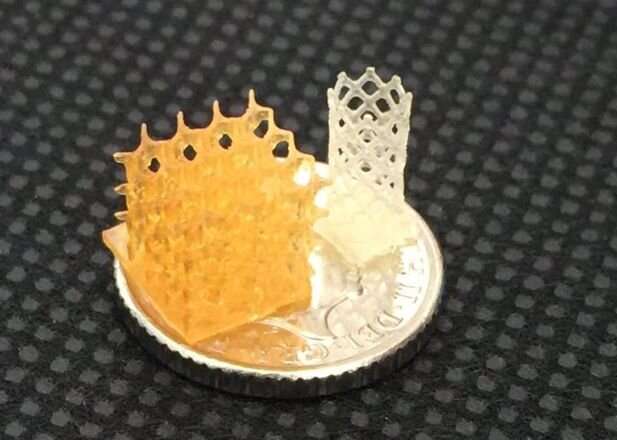
Research published today has demonstrated the viability of 3D-printed tissue scaffolds that harmlessly degrade while promoting tissue regeneration following implantation.
The scaffolds showed highly promising tissue-healing performance, including the ability to support cell migration, the ‘ingrowth’ of tissues, and revascularisation (blood vessel growth).
Professor Andrew Dove, from the University of Birmingham’s School of Chemistry, led the research group and is the lead author on the paper published in Nature Communications, which characterises the physical properties of the scaffolds, and explains how their ‘shape memory’ is key to promoting tissue regeneration.
Professor Dove commented: “The scaffolds have evenly distributed and interconnected pores that allow diffusion of nutrients from surrounding tissues. The shape memory means this structure is retained when the scaffold is implanted into tissues, and this supports the infiltration of cells into the scaffold while encouraging tissue regeneration and revascularisation.”
The scaffolds were created using 3D printing resin ‘inks’ developed during a major programme of biomaterials research led by Professor Andrew Dove at the University of Birmingham and Warwick University. The resins are being commercialised under the tradename 4Degra by 4D Biomaterials, a spinout from University of Birmingham Enterprise and Warwick Innovations that was launched in May 2020.
The scaffolds showed several major advantages over current approaches used to fill soft tissue voids that remain after trauma or surgery, including sufficient elasticity to conform to irregular spaces, the ability to undergo compression of up to 85% before returning to their original geometry, compatibility with tissues, and non-toxic biodegradation.
The paper describes several compositions for the 4Degra resins that enable materials of a wide range of strengths to be manufactured. All of the compositions include a photoinitiator and a photoinhibitor to ensure the resins rapidly turn into gel on exposure to light in the visible spectrum to enable their 3D printing into a range of scaffold geometries.
The researchers showed that the materials were non toxic to cells and they also performed mechanical testing to ensure the scaffolds could regain their shape, geometry and pore size after compression, and performed tests that showed the scaffolds can fill an irregular shaped void in alginate gel which was used as a mimic of soft tissue.
Laboratory studies demonstrated that the scaffold degrades by surface erosion into non-acidic products, which means the scaffold structure allows for slow, continuous tissue infiltration.
The findings were confirmed in a mouse model that simulates implantation into adipose (fat) tissue. These studies showed infiltration of adipocytes and fibroblasts and vascularisation at two months, and a tissue arrangement and macrophage presence that was indicative of normal tissue restoration rather than damaged, scarred tissue or an inflammatory response.
At four months, the researchers found small, mature blood vessels in the surrounding tissue. The scaffolds also demonstrated excellent biocompatibility. The collagen capsule formed around implants was less than 200 μm thick, which is well below the 500 μm threshold used for biocompatibility in other studies, and there was no calcification or necrosis.
Also at four months, 80% of the scaffold was still present, demonstrating the slow degradation predicted by the laboratory studies, and indicating the scaffolds would provide support for more than a year, allowing sufficient time for mature tissue ingrowth. The controls, which used poly(L-lactic acid) (PLLA) as a comparator, did not show a significant reduction over the four month period.
Professor Dove comments: “3D printed materials have received a lot of attention in the tissue engineering world. However void-filling materials to provide mechanical support, biocompatibility, and surface erosion characteristics that ensure consistent tissue support during the healing process, and this means a fourth dimension (time) needs to be considered in material design.
“We have demonstrated that it’s possible to produce highly porous scaffolds with shape memory, and our processes and materials will enable production of self-fitting scaffolds that take on soft tissue void geometry in a minimally invasive surgery without deforming or applying pressure to the surrounding tissues. Over time, the scaffold erodes with minimal swelling, allowing slow continuous tissue infiltration without mechanical degradation.”
4D Biomaterials has made fast progress in scaling up production of the 4Degra resin-inks at its laboratory in MediCity, Nottingham (UK) and is now offering technical grade material for commercial supply to 3D printing companies and medical device manufacturers.
Source: Read Full Article