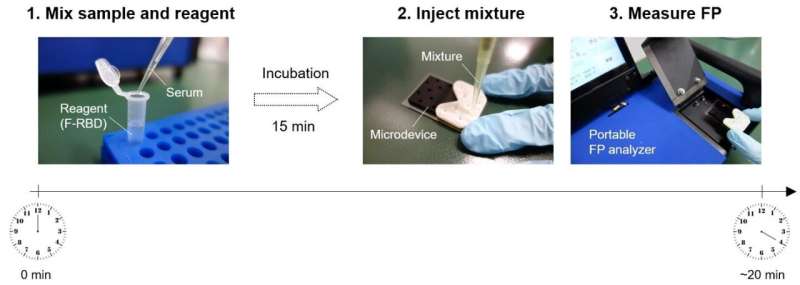
Scientists have developed a rapid, highly-accurate test to detect antibodies against the spike protein of SARS-CoV-2 in human serum, opening a new avenue for understanding the full extent of the pandemic and evaluating the effectiveness of vaccines.
In the 18 months since the emergence of COVID-19 pandemic, great strides have been made in discovering and inventing various approaches to track and control the spread of the SARS-CoV-2 virus. Rapid and accurate diagnosis has always been vital in this regard. The gold standard since the beginning of the pandemic has been the RT-PCR method; however, it is time-consuming, labor-intensive, and requires sophisticated equipment, and can only detect the presence of viral RNA in the samples.
A team of scientists from Japan, including Professor Manabu Tokeshi of Hokkaido University’s Faculty of Engineering, have developed a 20-minute test to detect and quantify antibodies against SARS-CoV-2 in human serum. Their findings were published in the journal Biosensors and Bioelectronics.
It is estimated that between 40% to 45% of individuals infected with SARS-CoV-2 are asymptomatic—despite being infected, they do not develop any symptoms of the disease. Identifying individuals who may have had asymptomatic COVID-19 is important to understanding the full extent of the pandemic. RT-PCR can only detect the presence of the viral RNA in samples; individuals who have recovered from the pandemic will only have antibodies to the virus, which RT-PCR cannot detect.
There are commercially available tests to detect the presence of antibodies against SARS-CoV-2; however, the majority of these tests are inaccurate. Lateral flow immunoassay is one of the most common tests for diagnosis of COVID-19 based on antibody detection. While rapid, it is a qualitative method, and cannot be used for the quantification of antibodies. Further, the method does not scale well, resulting in a low cost efficiency.
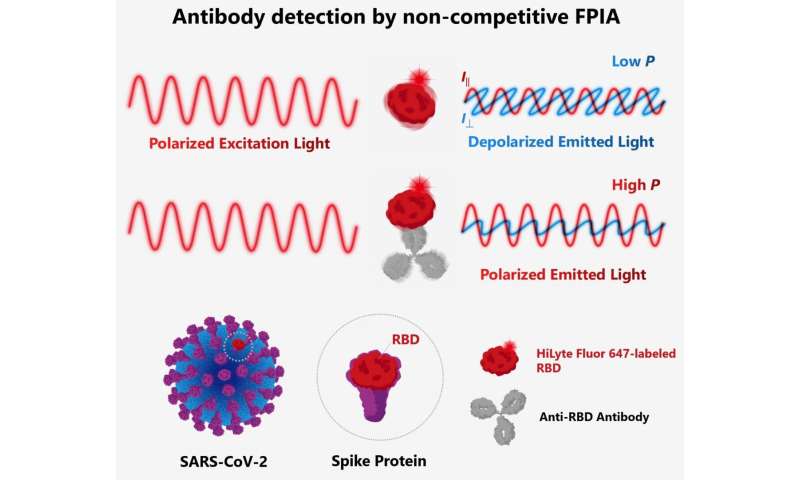
The scientists developed a method of detecting antibodies against SARS-CoV-2 in human serum using a method called non-competitive fluorescence polarization immunoassay (FPIA). This method is not just rapid, but can also be used to quantify the antibodies. FPIA has been used in the food and medical industry; the team has previously made many innovations to the method and associated equipment, including the development of a portable fluorescence polarization analyzer.
FPIA requires fluorescently-labeled recombinant SARS-CoV-2 spike proteins (F-RBD) and human serum to be mixed together for the test. Individuals who have been infected with or vaccinated against SARS-CoV-2 will have anti-spike protein antibodies in their serum. When these antibodies bind to F-RBD, polarized light is emitted, while F-RBD alone emits depolarized light. By measuring the degree of polarization using a fluorescence polarization analyser, the concentration of antibodies can be determined. The scientists optimized the test and evaluated it using samples of human serum from individuals diagnosed with COVID-19 and from those who had not been infected by SARS-CoV-2.
Source: Read Full Article