Cancer is a devasting disease that significantly affects a patient’s daily quality of life and well-being. Health care providers are increasingly aware that the everyday struggles that cancer patients must endure can negatively impact long-term survival. As a result, providers now recognize the importance of measuring patient-reported outcomes by asking directly about patient health and well-being.
Moffitt Cancer Center researchers have developed a tool to determine how a new class of prostate cancer therapies called radionuclide therapy (RNT) impacts patient-reported outcomes with the goal of using this information to guide treatment and improve quality of care. Their findings have been published in The Journal of Nuclear Medicine.
The use of patient-reported outcomes allows patients, caregivers and clinicians to better understand how cancer and its treatment impact quality of life, making cancer care more patient-oriented. The data are measured using patient questionnaires that focus on cancer symptoms, side effects of treatment, and social and emotional well-being.
The U.S. Food & Drug Administration encourages the addition of patient-reported outcomes measures in clinical trials. These measures are being incorporated into clinical practice, and results are being used to guide clinical decision-making and treatment planning.
The Moffitt team wanted to determine how a new type of therapy for patients with advanced prostate cancer affects quality of life. RNT is a new type of radiation therapy that links a radioactive chemical, called a radionuclide, to a cell-targeting molecule. Once injected into the body, the cell-targeting molecule can seek out prostate cancer cells, and the radionuclide “payload” irradiates and kills nearby cells.
Since RNT is a relatively new treatment for prostate cancer patients and has side effects that differ from standard therapy, Moffitt researchers wanted to understand which patient-reported outcomes are most influential for patients receiving RNT.
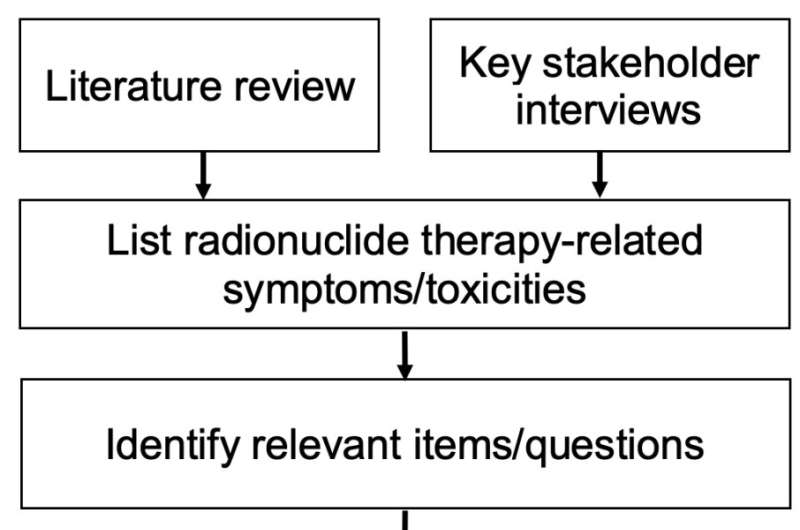
Moffitt researchers set out to develop a new patient-reported outcomes questionnaire that could accurately identify the key symptoms and side effects experienced by prostate cancer patients receiving RNT. Researchers reviewed the literature of published RNT clinical trials to create a list of symptoms and side effects associated with RNT. The research team also interviewed prostate cancer patients, caregivers, and clinicians to identify the most concerning symptoms and side effects.
“We were thorough in determining which symptoms and side effects are important to patients receiving RNT for prostate cancer,” said Lisa M. Gudenkauf, Ph.D., lead study author and research scientist in the Department of Health Outcomes and Behavior at Moffitt.
Their questionnaire, called the Functional Assessment of Cancer Therapy—Radionuclide Therapy (FACT-RNT), includes 15 questions to identify symptoms and side effects associated with RNT, such as dry mouth, nausea, loss of appetite, bone pain, fatigue and feelings of isolation due to illness/treatment.
The Moffitt research team hopes this newly developed assessment tool will help patients, their caregivers, and clinicians understand the effects of RNT, improve patient outcomes, and lead to more patient-centered care.
“The FACT-RNT will help future researchers better understand the impact of RNT and other therapies on quality of life in patients with prostate cancer,” explained Brian Gonzalez, Ph.D., senior study author and associate member of the Department of Health Outcomes and Behavior at Moffitt.
More information:
Lisa M. Gudenkauf et al, Developing a novel patient reported outcomes measure for prostate cancer patients receiving radionuclide therapy, Journal of Nuclear Medicine (2023). DOI: 10.2967/jnumed.122.264946
Journal information:
Journal of Nuclear Medicine
Source: Read Full Article