Squalene is primarily obtained from deep-sea shark liver oil, which accounts for 40–70% of the liver mass. Annually, three to six million deep-sea sharks are killed for squalene due to its human health benefits, which has a massive negative impact on marine ecosystems.
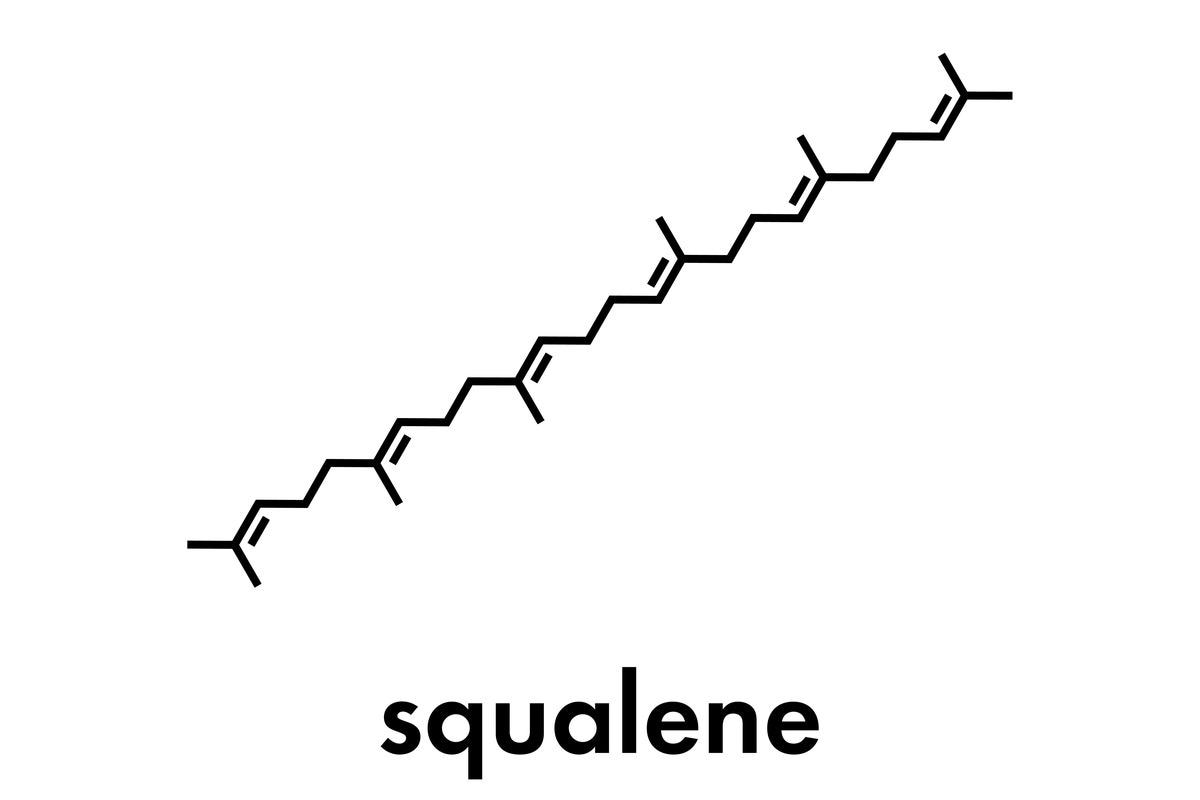
To address this unsustainable approach, microbial sources of squalene could provide a credible solution to plant- or animal-based squalene, albeit only a few bacteria have been discovered that can synthesize up to 30% squalene from dry biomass using native biochemical pathways. These squalene biosynthetic pathways, on the other hand, can be genetically modified to turn microorganisms into squalene-overproducing 'cellular factories.'
Background
Squalene in microorganisms is a key factor in the generation of several sterols, such as ergosterol. At room temperature, squalene is a liquid that is released via the skin. It is a powerful natural antioxidant that prevents lipid peroxidation, which protects cells from free radicals and reactive oxygen species.
It has also been shown to suppress carcinogenesis in the colon, lungs, and skin, as well as having chemoprotective and immunostimulatory effects, which has piqued the interest of the medical and pharmaceutical industries.
Squalene is the most prevalent component in the skin, along with polyunsaturated fatty acids, and is often utilized in skincare products for its antioxidant and moisturizing characteristics.
Squalene is found in trace amounts in most foods, except for a few plant species. Olive oil has a greater concentration of squalene when compared to other crops, with 200–700 mg/100 g of biomass. Amaranth seeds are another valuable source of squalene, however, only 4–6% of the seed biomass is oil. Furthermore, before being harvested, plant and animal sources must mature, which can take anywhere from 6 months to a year. As a result, squalene productivity from plant sources is quite low.
Squalene biosynthesis has also been discovered in yeasts and fungi. On a per cell dry weight (CDW) basis, the yeast and fungi species Saccharomyces cerevisiae, Torulaspora delbrueckii, Aspergillus nidulans, and Saccharomyces uvarum synthesize 1.6, 0.24, 0.3, and 14.3 mg/g squalene, respectively. Sugar substrates allow Thraustochytrids to acquire squalene at a rate of up to 316.64 mg/g CDW.
Previous research suggests that optimizing bioprocess variables such as media and cultivation conditions can significantly improve cell proliferation and squalene content/yield in thraustochytrid strains. Furthermore, it appears that genetically modifying bacteria is the only technique to make squalene on a large scale.
The study
In a recent review published in Trends in Biotechnology, a team of authors examined possible genetic engineering approaches that could be utilized to tailor the metabolic processes of yeasts, bacteria, and thraustochytrids to optimize the synthesis of squalene.
The formation of squalene
Depending on the type of microbe, squalene can be produced via two distinct processes. The 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway is used by bacteria and cyanobacteria, whereas the mevalonate (MVA) system is used by eukaryotes such as yeast, fungus, microalgae, thraustochytrids, plants, and animals.
The MVA pathway is regulated by a series of enzymes, of which 3-hydroxy-3-methylglutaryl-CoA synthase is the first in the process and is required for the conversion of acetyl-CoA to 3-hydroxy-3-methylglutaryl-CoA, which is then reduced to mevalonate by 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR).
Phosphorylation of mevalonate then occurs, resulting in mevalonate-5-phosphate, then mevalonate kinase further transforms it to mevalonate-5-pyrophosphate. Then, mevalonate-5-pyrophosphate is decarboxylated to form isopentyl diphosphate (IPP) by the enzyme IPP synthase, which also isomerizes IPP into dimethylallyl diphosphate (DMAPP). The condensing of DMAPP and IPP by farnesyl pyrophosphate (FPP) synthase generates geranyl diphosphate and FPP, which ultimately forms squalene.
Increasing squalene concentration in cyanobacteria and microalgae
One of the most well-known microalgal species capable of producing squalene is Botryococcus braunii. Several attempts to collect hydrocarbons and oils from this organism have been made, but these attempts have been hampered by the algae's slow development and limited genetic mechanisms. The suspected enzymes involved in the synthesis of squalene, on the other hand, can be used for heterologous expression in other strains to increase squalene output.
Other cyanobacteria have also been genetically modified to increase squalene output in several previous studies. A cyanobacterium, Synechocystis sp. PCC 6803, was engineered to make squalene from CO2. Squalene is produced in this organism via the MEP route for terpenoid biosynthesis, and it is then used for hopanoid formation by the enzyme squalene hopene cyclase (Shc).
Enhancing thraustochytrid squalene synthesis via biochemical engineering
Aurantiochytrium sp. Yonez 5-1 and Aurantiochytrium sp. 18W-13a are two thraustochytrid strains that have been identified as potential candidates for squalene production. Previous research conducted on these strains revealed that when cultured in a specific medium, Aurantiochytrium sp. 18W-13a produced 198 mg/gCDW squalene and Aurantiochytrium sp. Yonez 5-1 produced 316.64 mg/gCDW squalene.
Procedures for separating squalene from other lipids prior to further processing must be developed to enable its use in the relevant sectors. Few strategies for isolating squalene from total lipids, such as fraction crystallisation and saponification, have been documented in the literature.
Squalene separation from freeze-dried cells has previously been reported after saponification and extraction with n-hexane, with the use of a fractional crystallization technique in which the total isolated lipids were combined with methanol/acetone and kept at 20oC for 30 hours, then filtered and washed with chloroform and methanol. Based on these findings, strategies for separating squalene from total retrieved lipids and repurposing the residual lipids will be required for the procedure to be economically viable.
Implications
While current research has concentrated on optimizing bioprocesses to increase thraustochytrid squalene production, only yeast species have been investigated for metabolic engineering to increase squalene content.
To create viable metabolic engineering solutions, a deeper knowledge of the biosynthetic genes and pathways of squalene formation in thraustochytrids would be required.
- Alok Patel, Maurizio Bettiga, Ulrika Rova, Paul Christakopoulos, &Leonidas Matsakas. (2022). Microbial genetic engineering approach to replace shark livering for squalene. Trends in Biotechnology. doi: https://doi.org/10.1016/j.tibtech.2022.03.008 https://www.sciencedirect.com/science/article/pii/S0167779922000762
Posted in: Medical Science News | Miscellaneous News
Tags: Antioxidant, Bacteria, Carcinogenesis, Cell, Cell Proliferation, Cyanobacteria, Enzyme, Eukaryotes, Fatty Acids, Free Radicals, fungi, Genes, Genetic, Genetic Engineering, Kinase, Lipids, Liver, Lungs, Methanol, Olive Oil, Oxygen, Phosphorylation, Proliferation, Research, Saccharomyces Cerevisiae, Skin, Skincare, Yeast
.jpg)
Written by
Colin Lightfoot
Colin graduated from the University of Chester with a B.Sc. in Biomedical Science in 2020. Since completing his undergraduate degree, he worked for NHS England as an Associate Practitioner, responsible for testing inpatients for COVID-19 on admission.
Source: Read Full Article