The exchange of a single amino acid building block in a metabolic enzyme can lead to cancer. In addition, it can impair the immune system, according to scientists from the German Cancer Research Center (DKFZ), the University Hospitals in Mannheim and Heidelberg, and the German Cancer Consortium. It thus blocks the body’s immune response in the battle against the mutant molecule and also impedes immunotherapy against brain cancer. This finding opens new insights into cancer development and progression and it also suggests that rethinking antitumor immunotherapy is required.
Alterations in the genetic material are often the first step in carcinogenesis. In many cases, an exchange of a single amino acid building block is all it takes. A prime example for this is the mutant form of a metabolic enzyme called IDH1 (isocitrate dehydrogenase 1). This enzyme has an important task in cellular energy metabolism. However, if IDH1 has undergone an alteration at a specific site, it leads to the formation of a substance called 2 HG, which harms the body. It disturbs the metabolism and stimulates cell division, thus laying the cornerstone for cancer. The scientific term for a cancerogenic metabolic product like 2-HG is “oncometabolite.” 2-HG accounts for more than 70 percent of all low-grade gliomas, a type of brain cancer.
Scientists from the German Cancer Research Center and the University Hospitals in Mannheim and Heidelberg have now found that the oncometabolite additionally impairs the body’s immune response. Normally, the immune system recognizes mutant IDH1 as foreign. The altered molecule in the tumor should therefore attract immune cells. Based on this finding, scientists already developed a vaccine that sensitizes the immune system for the battle against brain tumors exhibiting the special IDH1 mutation.
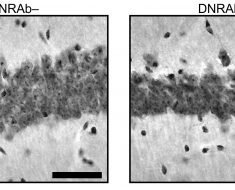
However, the opposite is the case, according to Michael Platten, a neurologist who leads a research department at the DKFZ and is director of the Neurology Department of University Medicine Mannheim (UMM). “In the immediate environment of tumors with the specific mutation in IDH1, we find only very small quantities of immune cells, which are additionally impaired in their functioning,” Platten said. “This made us curious and we aimed to find out whether and how the 2-HG oncometabolite directly influences the immune system.”
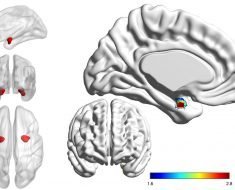
The investigators discovered that the tumor cells release 2-HG into their environment. T cells—immune cells of the body with an important role in the fight against cancer cells—take up released 2-HG. This subsequently blocks important signaling pathways in the T cells and the immune cells are re-programmed from an active to an inactive state.
“This might explain why the immune system fails to suppress the development of these tumors even though it is essentially capable of fighting tumor cells with the mutant IDH1 molecule,” said Lukas Bunse, DKFZ and Heidelberg University Hospital, who is one of the first authors of the publication in Nature Medicine. However, the scientists have also found a method to avoid this blockade. They administered an inhibitor developed by the team led by DKFZ researcher Andreas Deimling to mice with IDH1 mutant tumors. The inhibitor blocks the mutant IDH1 molecule so that no 2-HG forms in the tumor cells. Subsequently, the investigators in fact found larger quantities of active immune cells in the tumors and their immediate environment. In addition, immunotherapy combined with the inhibitor was substantially more effective.
Platten thinks that this finding has potential of learning more about other tumors and their treatment. “We now know several of these oncometabolites in different tumor types,” the neuroimmunologist said. “It would be interesting to investigate whether suppression of the immune response might be a higher principle in oncometabolites.”
Source: Read Full Article