In a new study researchers from the Institute for Experimental Pediatric Endocrinology of the Charité — Universitätsmedizin Berlin have successfully treat patients whose obesity is caused by a genetic defect. Aside from its beneficial effects on the patients, the researchers also provided insights into the fundamental signaling pathways regulating satiety of the new drug. The results of this research have been published in Nature Medicine.
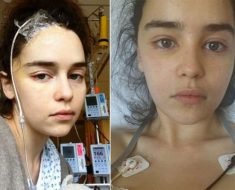
A mutation in the gene encoding the leptin receptor (LEPR) can cause extreme hunger starting with the first months of life. As a result, affected individuals develop extreme obesity during childhood. Increased exercise and reduced caloric intake are usually insufficient to stabilize body-weight. In many cases, obesity surgery fails to deliver any benefits, meaning that a drug-based treatment approach becomes increasingly important.
Two years ago, Dr. Peter Kühnen and the working group successfully demonstrated that treatment with a peptide, which activates the melanocortin 4 receptor (MC4R) could play a central role in the body’s energy metabolism and body weight regulation. Leptin, which is also known as the satiety (or starvation) hormone, normally binds to the LEPR, triggering a series of steps that leads to the production of melanocyte-stimulating hormone (MSH). The act of MSH by binding to its receptor, the melanocortin 4 receptor (MC4R) which transduce the satiety signal to the body. However, if the LEPR is defective, the signaling cascade is interrupted. The patient’s hunger remains unabated, placing them at greater risk of becoming obese. As part of this current study, researchers used a peptide that binds to the MC4R in the brain, and this activation trigger the normal satiety signal. Working in cooperation with the Clinical Research Unit at the Berlin Institute of Health (BIH), the researchers were able to record significant weight loss in patients with genetic defects affecting the LEPR.
“We also wanted to determine why the used peptide was so effective and why, in contrast to other preparations with a similar mode of action, it did not produce any severe side effects,” explains Dr. Kühnen. “We were able to demonstrate that this treatment leads to the activation of a specific and important signaling pathway, whose significance had previously been underestimated.” Dr. Kühnen’s team is planning to conduct further research to determine whether other patients might benefit from this drug: “It is possible that other groups of patients with dysfunctions affecting the same signaling pathway might be suitable candidates for this treatment.”
Source: Read Full Article